Abstract
Nineteen populations of Sphagnum affine were included in a study of genetic diversity and structure in fragmented and less fragmented landscapes, and differentiation at intercontinental and three regional levels. Isozyme electrophoresis of eight enzyme systems revealed 12 variable loci, which could be used for haplotype identification. A hierachical analysis of variance (AMOVA) revealed no significant intercontinental differentiation, and very limited differentiation among European regions. A trend of decreasing diversity with increasing latitude was apparent. Gametic phase disequilibria was high, suggesting nonrandom mating and regionally high incidences of inbreeding. The partitioning of genetic variation within and among populations in each region varied among regions, the northernmost populations having 86% of the total variation among populations, the southernmost in Scandinavia having 25% of the variation among populations, whereas the American populations displayed 89% of the variation within populations. Fifteen alleles at eight loci occurred in the U.S.A. which were not encountered in Europe, whereas only three European alleles at one locus in three populations were not encountered in U.S.A.
The differences in diversity between North America and Europe may result from loss of genetic diversity caused by founder effects during postglacial recolonization of northern Europe. In Europe, the main mountain ranges extend E–W, posing severe barriers to northwards migration of lowland species, compared to the N–S trend of mountain ranges in North America. The decline in genetic diversity and increase in population differentiation and gametic phase disequilibria towards the north in Scandinavia may be caused by a series of founder effects during postglacial migration. These may have corresponded to minor climatic oscillations that influenced the migration front/leading edge in the suboceanic lowlands of Norway. According to this model random genetic drift will be an increasingly important structuring factor with latitude.
Similar content being viewed by others
Introduction
Anthropogenic fragmentation of landscapes has taken place in all parts of Europe, but some of the northernmost areas are less disturbed by human activities, leaving large areas in a natural or seminatural state. In central Norway, large mire areas remain and the landscape is generally less influenced by agriculture than landscapes in southernmost Scandinavia. The landscape of southern Sweden is generally the more fragmented, with mires scattered in an agricultural and urban landscape. The significance of habitat fragmentation on present day genetic diversity in populations of bryophytes has attracted little attention. Theory predicts that small isolated populations will potentially experience a high degree of random genetic drift, which can be enforced by high incidences of inbreeding, and in some cases the probability of gene flow among populations may be affected (Young et al., 1996). Populations in which genetic drift predominates are more prone to local extinction than populations without genetic drift because of the loss of genetic variation (Barrett & Kohn, 1991).
Sphagnum affine Ren. & Card. is a haploid, dioecious, occasionally to rarely sexually reproducing, lowland species with an ability to grow laterally by bifurcation. Formerly included in S. imbricatum Hornsch. ex Russ., S. affine received little attention until Flatberg (1984) resurrected it. The species is amphiatlantic with suboceanic tendencies, and rarely occurs in exposed eu-oceanic habitats (Flatberg, 1986). In Europe, S. affine grows in a variety of mesotrophic fens, but may also occur in more oligotrophic mires. It is considered dependent on a rather warm vegetation season (Flatberg, 1986). In North America, it occurs in forested mires, e.g. Cedar swamps and in open mire habitats similar to those in Europe. It avoids ombrotrophic mires, but may be found in poor minerotrophic fen-soaks, between ombrotrophic parts of larger mire-complexes (Table 1). In North America, S. affine extends northwards to Newfoundland, whereas the northernmost populations in Europe are found scattered along the west coast of Norway as far north as Meløy (66°43′N; Flatberg, 1986). Many bryophytes are distributed widely and display large disjunctions. Disjunct distribution patterns may be caused by repeated long distance dispersal events, past geological events or separation of populations during glaciations. The distribution of genetic variation in intercontinentally disjunct populations may provide important information about evolutionary rates/ages of species, as well as postglacial migration patterns. The present study is aimed at: (i) assessing the distribution of genetic variation in S. affine in fragmented and less fragmented landscapes along a S–N gradient, i.e. whether populations from southern Sweden are less diverse and more influenced by predicted population genetic consequences of habitat fragmentation than populations further north; and (ii) investigating whether populations on either side of the Atlantic Ocean are genetically differentiated.
Materials and methods
Sampling
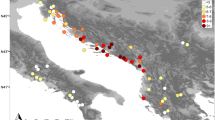
Gametophytes of Sphagnum affine were collected from mire habitats in four geographical regions on two continents; central Norway, southern Norway, southern Sweden in Europe and New Jersey in U.S.A. (Fig. 1). A total of 640 samples from 19 populations were included (Table 1). Samples were collected every 2 m along a transect, and on each mire, 30–50 samples were collected. However, in some cases it was not possible to follow a transect owing to mire topography or vegetation structure. The 2 m separation between individual samples was never violated, nor were very small populations included where there was a possibility of sampling fewer than 15 samples. Plants were cultivated prior to electrophoresis in order to bring them into a vigorous condition.
Enzyme assays
Extractions were performed using a pestle on cooled porcelain plates. Thirty microlitres of extraction buffer (Mitton et al., 1979; modification according to Cronberg, 1995) was added per capitulum sample, after rinsing in distilled water and removing excess water. Extracts were absorbed onto paper wicks (Whatman no. 3) and stored in microtitre dishes at −80 °C until electrophoresis. Electrophoresis was performed on 11% horizontal starch gels. Sodium- citrate (pH 7.0)/sodium-histidine (pH 7.0) buffer (Soltis et al., 1983; no. 1) was used to screen aconitase (ACO, EC 4.2.1.3), isocitrate dehydrogenase (NADP) (IDH, EC 1.1.1.42), phosphoglucomutase (PGM, EC 5.4.2.2) and shikimate dehydrogenase (SKD, EC 1.1.1.25). Sodium borate (pH 8.0)/tris-citrate (pH 8.6) buffer (Wendel & Weeden, 1989; no. 5) was used to resolve alcohol dehydrogenase (NAD) (ADH, EC 1.1.1.1), asparatate aminotransferase (AAT, EC 2.6.1.1), formate dehydrogenase (FDH, EC 1.2.1.2) and glucose-6-phosphate isomerase (GPI, EC 5.3.1.9).
Staining methods for ADH, FDH, GPI, IDH, PGM, PGI, SKD followed Cronberg (1995), with some minor modifications of the amounts of substrates and cofactors. AAT and ACO were stained according to Wendel & Weeden (1989). Details of electrophoresis, extraction and the nomenclature of loci and alleles are described in Thingsgaard (in press).
Data analysis
Banding patterns were interpreted in terms of putative discrete loci with haploid expression. Haplotypes and allele frequencies were tabulated and used for analysis of genetic diversity and structure. Gene diversity (Nei, 1987), percentage of polymorphic loci (P; a locus was considered polymorphic if more than one allele was encountered), mean number of alleles per locus (A) and number of haplotypes were calculated for individual populations and as means over regions. The proportion of distinguishable genotypes (PD) sensu Ellstrand & Roose (1987) was calculated as an estimate of clonal diversity. The distribution of private alleles, i.e. alleles only found in one population was determined.
Quantification of genetic structure was carried out for a simple structure of haploid individuals within populations, as well as for hierarchical structures grouping populations according to continents or regions. Intercontinental differentiation was compared among populations in Europe and North America, respectively. Moreover, differentiation among predefined regions in Europe, namely central Norway, southern Norway and southern Sweden, respectively, was assessed (Fig. 1). The F-statistics were derived from an analysis of molecular variance (AMOVA) (Excoffier et al., 1992; Weir, 1996). The significance of the F-statistics was tested using a nonparametric method described in Excoffier et al. (1992).
Linkage disequilibria between all pairs of loci was tested by the exact test of linkage disequilibrium described in Slatkin (1994) and Schneider et al. (1997). The proportion of linked loci (Pd) relative to the maximum number of loci (Stenøien & Såstad, 1999) was estimated for each population. Gametic phase equilibria were calculated from haplotypic data; thus, the limited number of haplotypes encountered in most European populations renders the information obtained less straightforward to interpret than if more haplotypes were found in populations. This measure is included, despite the long overlapping generations, as it gives an indication of the relative amount of recombination taking place in the populations.
The calculations of standard genetic diversity statistics, F-statistics, AMOVA and test for linkage disequilibria were performed using ARLEQUIN v.1.1 (Schneider et al., 1997).
Results
Electrophoretic patterns
Twelve putative loci with haploid expression were resolved, all of which were polymorphic in at least one of the surveyed populations. All loci, except for Aat-3, displayed the expected haploid banding patterns. In addition, Idh-1 consistently produced one intensely staining band and a fainter band migrating at a relative mobility identical to the other. The latter band was treated as a ‘ghost band’. Aat-3 showed the three-banded duplication-pattern found in all other species of Sphagnum section Sphagnum so far surveyed, which suggests the occurrence of an ancient duplication. However, due to variable resolution this locus was omitted from formal analysis. Detailed descriptions of banding patterns are presented elsewhere (Thingsgaard, in press).
Overall genetic diversity of Sphagnum affine populations
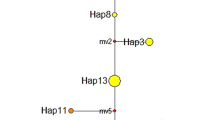
The mean frequency of polymorphic loci (P) in the total sample was 43% (range 0.0–92%; Table 2), the average gene diversity (Nei, 1987) was calculated as Hs=0.122 (range 0.00–0.31; Table 2) and the mean number of alleles per locus averaged 1.55 (range 1.00–2.58; Table 2). Ninety-five haplotypes were encountered across the 19 populations surveyed. The frequency distribution of haplotypes is skewed, as most haplotypes occur in a single or few populations. The commonest six haplotypes accounted for 51% of the total variation within the entire sample. The most widespread haplotype occurred in 68% of the populations surveyed. The proportion of distinguishable genotypes ranged from 0.04 to 0.67.
Private alleles at low frequency were encountered in 13 populations. Nine of the private alleles were found in the U.S.A., two in southern Sweden and one in southern Norway. Only one population (V01) in southern Sweden had a private allele with high frequency (63%; Table 3).
Genetic variability on different continents and in different regions
Those populations originating from North America were more variable for all diversity measures compared to the European populations. Mean genetic diversity (Nei, 1987) over loci (Hs) was estimated to 0.289 compared to a European mean of Hs=0.098. Mean number of alleles per locus (A) was 2.3 in U.S.A. compared with 1.4 in Europe. Within Europe, pronounced differences exist among regions in terms of diversity measures. The southernmost populations (southern Sweden) were on average more variable (Hs=0.162) than the northernmost populations (central Norway) (Hs=0.029). The same trend is apparent for all diversity measures (Table 2). This might suggest clinal variation at one or more loci, but no such trend is apparent from allele frequency data (Table 3).
Clonal diversity, measured as the proportion of distinguishable genotypes (PD), was approximately three times larger in populations in southern Sweden than in central Norway. The southern Norwegian populations fall in between for all measures of diversity (Table 2). The clonal diversity (mean PD) encountered in the populations from U.S.A. was much higher than even the most diverse European region. Fifteen alleles at eight loci absent from the European sample were encountered in the three American populations (Table 3). Of these, one allele (Fdh-1,100) might represent a low-frequency, relict occurrence from an ancient hybridization event (Thingsgaard, in press). Only three European alleles, all sampled at Aat-2 from three of 16 populations, were absent in the three New Jersey populations. In addition, two alleles, possibly originating from very rare hybridization events between S. affine and S. austinii Sull. (Thingsgaard, in press), were unique to Europe.
Population differentiation at intercontinental and regional scale
No significant substructuring among populations, regions and continents was revealed (Table 4). The fixation index (FST=0.512) showed that the variation was partitioned almost equally among and within populations when the total sample is considered. Among European regions, differentiation was small (FRT=0.043), and a slightly larger fraction of the variation was found among populations compared to within European populations (Table 4).
The amount of genetic variation partitioned among populations within regions varied dramatically between regions. Most of the variation was distributed within rather than among populations in New Jersey Pine Barrens (FSTreg=0.105) and southern Sweden (FSTreg=0.248), whereas most of the variation was distributed among populations in southern Norway (FSTreg=0.660) and extremely so in the central Norwegian populations (FSTreg=0.864; Table 4; Fig. 2).
Estimate of recombination
Gametic phase disequilibria were encountered in 94% of the population samples; linkage disequilibria was absent in only one southern Swedish population (S3). The amount of linkage disequilibrium showed large differences among populations both within and among regions. The percentage of linked loci relative to the maximum number (Pd) estimated as a mean of each region increased with latitude (Table 5).
Discussion
Genetic diversity
The low genetic diversity in the northernmost populations of Sphagnum affine, as estimated by genetic diversity and clonal diversity measures, does not support a hypothesis of lower genetic diversity in more anthropogenically fragmented areas. The pattern is better explained by random genetic drift during postglacial recolonization. Assuming that Scandinavian populations of S. affine have colonized mires serially after migrating northwards from refugia in southern Europe at the onset of the Holocene or later, they may have experienced bottlenecks during minor climatic oscillations and lost genetic diversity towards the north. This genetic depletion includes the loss of the otherwise widespread allele Adh-1 120 in central Norwegian populations. It would have been interesting to include populations from unglaciated areas in southern Europe to assess whether they are more genetically variable than the populations included in this study. This has not been possible, mainly as a consequence of the rarity of S. affine south of the Scandinavian Peninsula, although reduction of genetic diversity with latitude suggests that southern populations may be more diverse.
A decrease of gene diversity with increasing latitude has been observed in the mosses Meesia triquetra (Richt.) Aongstr. in the western part of its range (Montagnes et al., 1993) and Leucodon sciuroides (Hedw.) Schwaegr. in unglaciated vs. glaciated parts of Europe (Cronberg, 2000), as well as in angiosperms (e.g. Broyles, 1998). In comparison to the related oceanic Sphagnum austinii, confined to lowland atlantic bogs in Europe (Flatberg, 1986) and almost devoid of genetic diversity (Thingsgaard, in press), S. affine is able to grow and survive in a diverse array of mire habitats. This may be the reason why it has survived the migration with more genetic diversity preserved. For the same reason it may have had more glacial refugia in southern Europe, possibly extending further eastwards than S. austinii. Likewise, suboceanic S. rubellum is reported with reduced levels of genetic variation in Scandinavia compared to Great Britain, but little genetic differentiation among lowland populations in Scandinavia (Cronberg, 1998). Sphagnum rubellum extends further north and east, it occurs in a wider range of mire habitats, and may not be as dependent on warm summer temperatures as S. affine (Flatberg, 1986). Cronberg (1998) speculates, that the dry climate during glaciations may have reduced the area available for survival of S. rubellum in southern Europe. It is likely that S. affine and S. rubellum shared refugia in southern Europe, and have faced similar constraints during postglacial migration. However, as S. affine is less well adapted for colder climates and has a slightly more oceanic affinity, it may have been less successful towards the north, where the effects of minor climatic oscillations were severe, than S. rubellum. Consequently, it has experienced more bottlenecks while colonizing Norway, and thus retained increasingly depleted populations with latitude. Similar patterns are reported for other species of animals and plants (Hewitt, 1999).
The generally higher amount of genetic diversity found in populations from New Jersey may be ascribed to the fact that the Coastal Plains, where the sample of American populations were collected, have not been glaciated during the Wisconsin glaciation (Karlin & Andrus, 1988). Comparisons of bryophytes from glaciated areas in North America and Europe show that in most cases the gene diversity in North America is higher than in Europe, i.e. glaciation does not necessarily imply that populations became genetically depleted in North America. Lower genetic diversity was reported for Plagiomnium medium (B.S.G.) T.Kop. in Europe compared to North America (Wyatt et al., 1992), and some evidence of the same trend may be observed in Sphagnum angustifolium (Russ.) C.Jens. Clonal diversity of S. angustifolium originating from Newfoundland (calculated from data in Stenøien & Såstad (1999)) is more than twice as high as in European populations, suggesting that the effect of glaciation on intrapopulation genetic diversity is notable in populations of Sphagnum in Europe compared to glaciated areas in North America. The main mountain ranges in central and southern Europe extend E–W and are a substantial barrier to north- and southward migration. In contrast, the mountain ranges in North America are orientated N–S, and thus similar barriers to N–S migrations of fauna and flora do not exist (Crum, 1972; Wyatt et al., 1993). A possible explanation of the higher genetic diversity found in North American populations of S. affine compared to European populations may be that the species has not gone through severe bottlenecks during glaciations and subsequent recolonizations to the same extent as in Europe. Recently, Såstad et al. (2000) reported lower genetic diversity in North America compared to Europe in limited samples of Sphagnum majus (Russ.) C.Jens from Newfoundland and New York. However, S. majus is a circumboreal species, only rarely extending to lowland nemoral (deciduous woodland) temperate areas. Moreover, it is rare in the western part of North America. More genetic diversity may have been preserved in this boreal species if it did not survive the glaciation in Europe in the same, probably very limited, southern refugia as S. affine and S. rubellum, but rather were able to survive in more and larger refugia located further south-east or east of the North European ice sheet than the oceanic and suboceanic species. This may also explain the rather high genetic diversity found in S. angustifolium and S. capillifolium (Ehrh.) Hedw., two species extending into continental and subalpine areas.
Differentiation at the intercontinental and regional levels
Little differentiation was found between European and North American S. affine populations. Similar patterns are reported in other studies of bryophytes from these areas (Wyatt et al., 1992; Stenøien & Såstad, 1999; Hanssen et al. 2000; Såstad et al., 2000).
Among European populations, Cronberg (1998) found low levels of differentiation in S. capillifolium and S. rubellum, indicating considerable gene flow. A low impact of processes causing population divergence was invoked by Stenøien & Såstad (1999) to explain the lack of intercontinental differentiation in S. angustifolium. However, in many clonal species the majority of genetic variation is often found among populations (Ellstrand & Roose, 1987), a pattern that is also found in S. affine. The genetic variation found in populations originating from southern and central Norway is strongly partitioned among populations (66% and 86%, respectively), suggesting a large impact of genetic drift (Table 4; Fig. 2). The lowest amount of interpopulation differentiation was found regionally in New Jersey (c. 10%) and southern Sweden (c. 25%). This suggests limited, but still higher, gene flow (past or present) between populations within these regions compared to the northernmost populations in central Norway.
The causal factors determining the genetic isolation among populations of S. affine in, for example, southern Sweden and central Norway, may be different in each region. The FSTreg estimates for central Norway are very high, which allows for associating the regional FSTreg value with a Nm value even though regional equilibrium between loss of alleles due to genetic drift and their replacement by gene flow among populations is not found (Hutchison & Templeton, 1999). The Nm value inferred via Wright’s (1931) equation:

from a mean regional FST value of 0.83 is 0.102, inferring that drift is a proportionately more important structuring agent than gene flow (Kimura & Weiss, 1964) in central Norwegian populations of S. affine. This may support an assumption that the effect of climatic fluctuations on the populations forming the northern distributional limit or ‘migration front’ is reflected in the size of the effective breeding population; hence, there is a greater susceptibility to genetic bottlenecks. Severe isolation of each finite population will cause random genetic drift to become relatively more important than gene flow. It is somewhat more difficult to interpret the southern Swedish region with respect to the individual effects of interpopulation gene flow and random genetic drift on the regional population structure. Sampling of geographically close populations has not been accomplished in this region, and it is not possible to assess whether the regional structure approaches equilibrium. It is very unlikely however, that southern Swedish populations approach a migration–drift equilibrium, because populations inhabiting landscapes undergoing anthropogenic changes causing habitat fragmentation are not expected to be at equilibrium (Whitlock & McCauley, 1998). Ancient gene flow among populations and/or possibly more colonization events from less depleted sources in the southern Sweden region, as well as a longer persistence of the conditions required for the populations to exist, compared to the Norwegian populations, may explain the comparatively lower differentiation among populations from Sweden compared to central Norway. Even if gene flow has had, or has, a more prominent role in population structuring in southern Sweden than in central Norway, interpopulation gene flow may generally be considered low among S. affine populations in Europe. This tendency increases with latitude, and may contribute to the partitioning of genetic variation among rather than within populations (Table 4). The existence of a private allele at high frequency at the locality near Ll. Lesjön in south-west Sweden (V01) indicates that present-day interpopulation gene flow is low. The same applies to two closely situated populations sampled in the same large mire complex in south-west Norway (H1 and H2), which are very different with respect to diversity. H1, the smaller sample, is more polymorphic, not only because of alleles occurring at low frequencies, but also because of two alleles occurring at intermediate frequency that are not represented in the nearby population. The proportion of distinguishable genotypes, i.e. the clonal diversity, is also higher at H1. Finally, the only mire site in the total sample, which housed a completely monomorphic population (T15) was situated at the island of Hitra, central Norway, only a few kilometres from another population which was variable at five loci (T16).
Recombination within populations
Some of the findings suggest that genetic recombination within populations is limited, and that it decreases with latitude. The great amount of gametic phase equilibria suggests that most populations are inbreeding, and are not panmictic. The increase in gametic phase disequilibria with latitude indicates that the northernmost populations have been established most recently through multiple colonizations. In combination with the rare production of sporophytes seen today (i.e. most reproduction may be clonal) this indicates that recombination has not been sufficient to break down the patterns of linkage disequilibria in the populations acquired during postglacial migration. When clonal size increases, the chance of successful recombination is reduced owing to limited gamete dispersal distances (McQueen, 1985; Cronberg, 1993; Daniels, 1993), unless male and female clones intermix, which they may. Even if sexual reproduction occurs, the chance that the male and female plant involved may have originated from the same sporophyte (autogamy) appears to be high, as most spores are assumed to be dispersed within a limited distance from the mother plant (McQueen, 1985). Establishment of sporelings in existing clonal populations of Sphagnum is hard to assess by direct means. Little is therefore known about the germination of Sphagnum spores in nature (Cronberg, 1993; Daniels, 1993). It would be interesting to know, whether a spore is able to germinate, a protonema develop, and a new individual to establish in a dense carpet of other mire Sphagnum species, or whether new establishment is largely dependent on disturbances creating suitable niches. A recent experimental study suggests that Sphagnum spores may not germinate in moss carpets, but natural recruitment from spores is dependent on disturbance as well as nutrient release from litter and coverage provided by vascular plants (Sundberg and Rydin, in press).
If many European populations of S. affine are not panmictic, and sexual reproduction only takes place occasionally and to some extent among closely related individuals with possibly low success of establishment, the amount of haplotypic variation found must be the result of gene flow in the past. Genetic drift has been playing an important role in the structuring of genetic variation in this species. The northernmost populations confined to the suboceanic lowland of Norway have almost certainly suffered more founder effects during postgalcial migration than the populations arriving on the Scandinavian Peninsula from glacial refugia in the south, and may possibly experience unfavourable breeding seasons due to climatic constraints. This will explain why populations in this region are more inbred, less diverse and more genetically isolated; a pattern expected in the more anthropogenically fragmented agricultural landscape in southern Sweden.
References
Barrett, S. C. H. and Kohn, J. R. (1991). Genetic and evolutionary consequences of small population size in plants: Implications for conservation. In: Falk, D. A. and Holsinger, K. E. (eds) Genetics and Conservation of Rare Plants. pp. 3–30. Oxford University Press, New York.
Broyles, S. (1998). Postglacial migration and the loss of allozyme variation in northern populations of Asclepias exaltata (Asclepiadaceae). Am J Bot, 85: 1091–1097.
Cronberg, N. (1993). Reproductive biology of Sphagnum. Lindbergia, 17: 69–82.
Cronberg, N. (1995). Isozyme electrophoresis in Sphagnum: an analysis of methodology. Lindbergia, 20: 40–48.
Cronberg, N. (1998). Population structure and interspecific differentiation of the peat moss sister species Sphagnum rubellum and S. capillifolium (Sphagnaceae) in Northern Europe. Pl Syst Evol, 209: 139–158.
Cronberg, N. (2000). Genetic diversity of the epiphytic bryophyte Leucodon sciuroides in formerly glaciated versus nonglaciated parts of Europe. Heredity, 84: 710–720.
Crum, H. (1972). The geographic origins of the mosses of North America’s eastern deciduous forest. J Hattori Bot Lab, 35: 269–298.
Daniels, R. E. (1993). Phenotypic and genotypic variation in Sphagnum. Adv Bryol, 5: 31–60.
Ellstrand, N. C. and Roose, M. L. (1987). Patterns of genotypic diversity in clonal plant species. Am J Bot, 74: 123–131.
Excoffier, L., Smouse, P. and Quattro, J. (1992). Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics, 131: 479–491.
Flatberg, K. I. (1984). A taxonomic revision of the Sphagnum imbricatum complex. K Norske Vid Selsk Skr, 3: 1–80.
Flatberg, K. I. (1986). Taxonomy, morphovariation, distribution and ecology of the Sphagnum imbricatum complex with main reference to Norway. Gunneria, 54: 1–118.
Hanssen, L., Såstad, S. M. and Flatberg, K. I. (2000). Population structure and taxonomy of Sphagnum cuspidatum and S. viride. Bryologist, 103: 93–103.
Hewitt, G. M. (1999). Post-glacial re-colonisation of European biota. Biol J Linn Soc, 68: 87–112.
Hutchison, D. W. and Templeton, A. R. (1999). Correlation of pairwise genetic and geographic distance measures: Inferring the relative influences of gene flow and drift on the distribution of genetic variability. Evolution, 53: 1898–1914.
Karlin, E. F. and Andrus, R. E. (1988). The Sphagnum species of New Jersey. Bull Torrey Bot Club, 115: 168–195.
Kimura, M. and Weiss, G. H. (1964). The ‘stepping stone’ model of population structure and the decrease of genetic correlation with distance. Genetics, 49: 561–576.
Mcqueen, C. B. (1985). Spatial pattern and gene flow distances in Sphagnum subtile. Bryologist, 88: 333–336.
Mitton, J. B., Linhart, Y. B., Sturgeon, K. B. and Hamrick, J. L. (1979). Allozyme polymorphisms detected in mature needle tissue of ponderosa pine. J Hered, 70: 86–89.
Montagnes, R. J. S., Bayer, R. J. and Vitt, D. H. (1993). Isozyme variation in the moss Meesia triquetra (Meesiaceae). J Hattori Bot Lab, 74: 155–170.
Nei, M. (1987). Molecular Evolutionary Genetics. Columbia University Press, New York.
Såstad, S. M., Flatberg, K. I. and Hanssen, L. (2000). Origin, taxonomy and population structure of the allopolyploid peat moss Sphagnum majus. Pl Syst Evol, 225: 73–84.
Schneider, S., Kueffer, J. M., Roessli, D. and Excoffier, L. (1997). Arlequin: A software for population genetic data analysis, Release 1.1. Genetics and Biometry Laboratory, University of Geneva, Geneva.
Slatkin, M. (1994). Linkage disequelibrium in growing and stable populations. Genetics, 137: 331–336.
Soltis, D. E., Haufler, C. H., Darrow, D. C. and Gastony, G. J. (1983). Starch gel electrophoresis of ferns: a compilation of grinding buffers, gel and electrode buffers and staining schedules. Am Fern J, 73: 9–27.
Stenøien, H. K. and Såstad, S. M. (1999). Genetic structure in three haploid peatmosses (Sphagnum). Heredity, 82: 391–400.
Sundberg, S. and Rydin, H. in press Habitat requirements for establishment of Sphagnum from spores. J Ecol. in press.
Thingsgaard, K. in press. Taxon delimitation and genetic similarities of the Sphagnum imbricatum – complex as revealed by isozyme electrophoresis. J Bryol.
Weir, B. S. (1996). Genetic Data Analysis, II. Sinauer Associates, Sunderland, MA.
Wendel, J. F. and Weeden, N. F. (1989). Visualization and interpretation of plant isozymes. In: Soltis, D. E. and Soltis, P. S. (eds) Isozymes in Plant Biology, pp. 5–45. Dioscorides Press, Portland, OR.
Whitlock, M. J. and McCauley, D. E. (1998). Indirect measures of gene flow and migration: Fst ≠ 1/(4Nm+1). Heredity, 82: 117–125.
Wright, S. (1931). Evolution in Mendelian populations. Genetics, 16: 97–159.
Wyatt, R., Odrzykosky, I. J. and Stoneburner, A. (1992). Isozyme evidence of reticulate evolution in mosses: Plagiomnium medium is an allopolyploid of P. ellipticum P. insigne. Syst Bot, 17: 532–550.
Wyatt, R., Odrzykosky, I. J. and Stoneburner, A. (1993). Isozyme evidence regarding the origins of the allopolyploid moss Plagiomnium curvatulum. Lindbergia, 18: 49–58.
Young, A., Boyle, T. and Brown, T. (1996). The population genetic consequences of habitat fragmentation in plants. Trends Ecol Evol, 11: 413–418.
Acknowledgements
This study was supported by grants from Mikroorganismefonden af 1988, Ingeniør Svend G. Fiedler and Hustru’s Legat and Mag. art. Marcus Lorenzens Legat, which are all gratefully acknowledged. I acknowledge permission granted by the Ecology Department at the Botanical Institute in Copenhagen to use the lab, and laboratory technician Ruth Bruus Jacobsen for tolerating Sphagnum (and me) in her lab. Länsstyrelsen, Jönköbing, granted permission to collect plants in the Komosse Nature Reserve. Hans K. Stenøien commented on a very early draft manuscript. I wish to thank Nils Cronberg and Sigurd M. Såstad for useful comments on later versions. Valuable information on locations of mires in southern Sweden was provided by Tomas Hallingbäck and Sven Fransson. Invaluable support was provided by Kjell Ivar Flatberg who accompanied me in the field on several occasions, placed topographic maps of central Norway at my disposal, as also gave many suggestions for localities in Norway and other issues related to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thingsgaard, K. Population structure and genetic diversity of the amphiatlantic haploid peatmoss Sphagnum affine (Sphagnopsida). Heredity 87, 485–496 (2001). https://doi.org/10.1046/j.1365-2540.2001.00939.x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1046/j.1365-2540.2001.00939.x
Keywords
This article is cited by
-
Genetic diversity and differentiation in Chinese sour cherry Prunus pseudocerasus Lindl., and its implications for conservation
Genetic Resources and Crop Evolution (2009)
-
Disjunct Occurrences of Plant Species in the Refugial Mires of Bulgaria
Folia Geobotanica (2009)
-
Genetic structure of Oryza rufipogon Griff. in China
Heredity (2008)
-
How to find the bogmoss, Sphagnum imbricatum s.l., in South Tyrol, Italy: Microscopically examine the Iceman’s colon contents
Vegetation History and Archaeobotany (2005)