Abstract
Allozyme electrophoresis was used to determine amount and structure of genetic variation within and between five congeneric haploid moss species: Polytrichum formosum, P. commune, P. uliginosum, P. piliferum and P. juniperinum. For the different species, gene diversity within populations (HS) ranged from very low (0.025) to moderate values (0.127), being, on average, lower than those observed for vascular plants and many other moss species. Polytrichum piliferum and P. juniperinum showed significantly higher levels of HS than the other species examined, which possibly might be explained by sexual reproduction being more prevalent in these two species, that often live in more dynamic habitats, where turnover of individuals is more frequent. Genetic variability was observed to be structured in contrasting ways at different levels. At the intraspecific level genetic differentiation among populations of most Polytrichum species was low, FST ≤ 0.1, indicating a considerable degree of gene flow by wind-dispersed spores over substantial distances. At the interspecific level strong divergence, genetic identities being on average I=0.222, was observed between most species studied, despite low levels of divergence at the morphological level. These I-values are significantly lower than observed for congeneric vascular plant species and most congeneric bryophyte species. This suggests that the morphological conservatism is not due to lack of genetic variability and evolutionary potential.
Similar content being viewed by others
Introduction
In contrast to a large number of investigations that have determined the amount and structure of genetic variation within and among vascular plant populations (Hamrick & Godt, 1990), bryophytes have received relatively little attention in this respect (Ennos, 1990). This seems undeserved as they are the second largest group of land plants and can be found in almost every terrestrial habitat and in many freshwater habitats, where they can reach high levels of abundance (During & Van Tooren, 1987). Moreover, bryophytes show various modes of reproduction: asexual reproduction via clonal growth and vegetative propagules, and sexual reproduction via spores produced by monoecious and dioecious species (During & Van Tooren, 1987; During, 1990). Because of this versatility in reproductive characteristics, they offer a unique opportunity to study the importance of different life histories on the level and structure of genetic variation (Ennos, 1990).
For vascular plants, it has been shown that breeding system, mode of reproduction, dispersal ability and spatial population structure significantly affect the structure and amount of genetic variation (Hamrick & Godt, 1990). Among the factors that are thought to shape the level and structure of genetic variation in bryophytes, many are related to reproductive biology (Longton, 1976; During, 1990; Ennos, 1990). Most of these reproductive characteristics of bryophytes are expected to decrease levels of genetic variation: predominance of asexual reproduction (e.g. During, 1990), high levels of self-fertilization in monoecious species (Longton, 1976; During, 1990; Ennos, 1990), rarity of sexual reproduction in dioecious species due to spatial separation of the sexes (Longton, 1976), and low regeneration probabilities of spores produced by sexual reproduction (e.g. Miles & Longton, 1987). In addition, most bryophytes show a predominantly haploid life cycle and consequently new recessive (slightly) deleterious mutations will not be concealed in heterozygotes but will be directly selected against (Longton, 1976; Ennos, 1990). Moreover, many forms of balancing selection, such as (marginal) overdominance, are not effective in haploids (Hoekstra, 1978). This possibly could explain the slow evolutionary change in morphology that has been observed for many bryophyte species, on the basis of which it has been assumed that since the Permian era speciation in bryophytes has occurred at a much lower rate than in vascular plants (Longton, 1976).
The few existing studies on allozyme variation in bryophytes (e.g. De Vries et al., 1983, 1989; Wyatt et al., 1989a; Derda & Wyatt, 1990; Hofman, 1991; Cronberg et al., 1997; Cronberg, 1998) do not particularly suggest low levels of genetic variation. On average, the level of genetic variation observed seemed not much lower than generally observed for vascular plants (Wyatt et al., 1989b). However, conspicuous differences in the amount of genetic variation have been observed between congeneric moss species, necessitating further investigations (Ennos, 1990).
The amount of genetic differentiation between populations is the result of the opposing forces of genetic drift and gene flow, respectively, promoting and constraining genetic differentiation. The degree of differentiation therefore can be a useful indicator of the amount of gene flow between (sub)populations (Wright, 1951). In mosses, gene flow is mediated by male gametes, spores, and asexual propagules or gametophyte fragments. Male gamete dispersal is very restricted and probably limited to a few centimetres (Longton, 1976), while spore dispersal appears to be strongly leptokurtic, most spores being deposited within a few metres from the source (e.g. Söderström & Herben, 1997). This would promote strong differentiation between populations. However, spores are produced in vast quantities, commonly exceeding a million spores per sporophyte in Polytrichaceae (Longton, 1997). Consequently, the small fraction of spores that disperses beyond the population borders, could still represent a considerable number. Moreover, spores possibly may disperse over very large distances, resulting in gene flow between geographically quite distant populations (Van Zanten & Pócs, 1981). However, as recruitment of new individuals from spores has been observed to be rare (e.g. Miles & Longton, 1987), effective gene flow by spores may still be very limited, notwithstanding substantial spore dispersal. Because many bryophyte species show strong clonal reproduction (During, 1990), this situation becomes even more complicated, making clear-cut predictions about expected levels of genetic differentiation rather difficult. Not surprisingly therefore both low (Cronberg et al. (1997) for Hylocomium splendens) and very high values (Wyatt (1992) for Plagiomnium ellipticum) of genetic differentiation among populations within species have been reported.
From a phylogenetic perspective, genetic differentiation between congeneric moss species has been observed to be large: genetic identities between vascular plant species have been found to be considerably higher than between bryophyte species (Wyatt et al., 1989b). This may indicate that the evolutionary rate is not constrained in bryophytes, but that morphological characters evolve at a different rate from allozymes.
To gain additional insights into the population biology of bryophytes and to investigate the effect of life history characteristics on genetic structure, we address in this paper several of these issues for five taxa of the moss genus Polytrichum: P. formosum, P. commune, P. uliginosum, P. piliferum and P. juniperinum. We use protein electrophoresis to estimate levels of genetic variation both within and among populations of these Polytrichum species from two different geographical origins. We discuss whether the differences in levels of genetic variation and genetic differentiation that we observed can be ascribed to differences in ecology or life history characteristics of the species. Finally, the level of genetic differentiation between the species is investigated and phylogenetic relationships are evaluated.
Materials and methods
The species
Five taxa of the moss genus Polytrichum (Hair-cap moss), P. formosum, P. piliferum, P. juniperinum, P. commune and P. uliginosum, have been investigated. The last two are often regarded as two infra-specific taxa of P. commune s.l. (P. commune var. commune and P. commune var. uliginosum Wallr.), but in this study we have treated them as two distinct species, because of the high level of genetic differentiation that has been observed between these two taxa (Bijlsma et al., 2000).
The five species are medium to large sized mosses and are all commonly found throughout Western Europe, and the Northern Hemisphere in general (Touw & Rubers, 1989), but prefer quite distinct habitats: P. piliferum and P. juniperinum are usually found in more disturbed and exposed, sandy habitats, whereas P. formosum, P. commune and P. uliginosum are generally found in more stable habitats, like acidic, humus-rich forest soils for P. formosum, wet peat bogs for P. uliginosum and relatively dry peat soils for P. commune (Touw & Rubers, 1989).
Phylogenetic relationships among Polytrichum species are still a matter of debate. Some authors have argued that P. formosum should be ascribed to a different genus Polytrichastrum instead of Polytrichum (Smith, 1971). Although generally haploid (n=7), for all species, in particular for P. formosum (Crum & Anderson, 1981), diploid specimens (n=14) have been observed (Fritsch, 1982), but in our study all populations of all species investigated seemed haploid and dioecious.
Sampling
Several Danish and Dutch populations were sampled for each of the five different species (see Table 1). To ensure that different individuals (genets) were sampled, samples were taken from clearly separated moss cushions at least two metres apart, and from each cushion one sample was taken as a small clump of neighbouring shoots. Samples were stored in plastic bags in the refrigerator (6°C) until electrophoresis.
Allozyme electrophoresis
Allozyme electrophoresis was performed for 11 enzyme systems for P. commune, P. uliginosum and P. formosum: aconitase (ACON, E.C. 4.2.1.3.), acid phosphatase (ACPH, E.C. 3.1.3.2.), alcohol dehydrogenase (ADH, E.C. 1.1.1.1.), glutamate-oxoacetate transaminase (GOT, E.C. 2.6.1.1.), glucophosphate isomerase (GPI, E.C. 5.3.1.9.), isocitrate dehydrogenase (IDH, E.C. 1.1.1.42.), malate dehydrogenase (MDH, E.C. 1.1.1.37.), 6-phosphogluconate dehydrogenase (6-PGD, E.C. 1.1.1.44.), phosphoglucomutase (PGM, E.C. 5.4.2.2.), shikimate dehydrogenase (SHDH, E.C. 1.1.1.25.) and triosephosphate isomerase (TPI, E.C. 5.3.1.1.). For P. piliferum and P. juniperinum, three additional enzyme systems were investigated: hexokinase (HK, E.C. 2.7.1.1.), malic enzyme (ME, E.C. 1.1.1.40.) and superoxide dismutase (SOD, E.C. 1.15.1.1.). The electrophoretic procedures, recipes for buffers and staining solutions are identical to those described in Bijlsma et al. (2000) and references therein. The staining solutions for HK, ME and SOD are described by Hofman (1991). The staining of HK and SOD was performed on gels ran on a Tris-borate EDTA pH 8.6 buffer-system, also described by Hofman (1991), whereas for ME a gel ran on a LiOH-borate pH 8.3 buffer-system was used (Bijlsma et al. 2000).
Data analysis
After interpretation of the banding patterns, multiple loci at one enzyme system were numbered (1–3), and a letter (a–h) was assigned to different alleles at a single locus (see Table 2). The enzyme systems ACPH, IDH and TPI showed complex banding patterns of three or more bands, that were difficult to interpret, and when variable, the electrophoretic mobility of several bands changed in concert. Therefore, these complex banding patterns were treated as a single locus. Because of inconsistent staining of locus Mdh-3 in P. juniperinum and P. piliferum and of locus Gpi-3 in P. commune, P. uliginosum and P. formosum, these loci were only scored in those species in which they could be consistently interpreted. In the phylogenetic comparisons involving all five Polytrichum species, only the 15 loci that had been analysed for all species were taken into account.
Allele frequencies were determined for all loci. Mean number of alleles per locus (A), percentage polymorphic loci (P) and mean gene diversity (HS; Nei, 1987) were calculated as measures of genetic variability within populations using BIOSYS (Swofford & Selander, 1981). F-statistics, FST and FCT, were calculated as measures of genetic differentiation among populations using ARLEQUIN (Schneider et al., 1997). Finally, Nei’s (1987) unbiased genetic identity (I) was calculated as a measure of genetic differentiation between species.
Results
Genetic variation within populations
Genetic variation could be scored at 16 putative loci for all populations of P. commune, P. uliginosum and P. formosum and at 21 putative loci for all populations of P. juniperinum and P. piliferum. Most enzyme loci showed a single band for each individual, and for polymorphic loci no heterozygotes were ever observed, confirming the haploid state of the species. Only 1–3 loci were polymorphic within each of the populations of P. commune, P. uliginosum and P. formosum (Table 1), and for some of these loci polymorphism was only due to rare alleles (i.e. alleles that were observed only once at these loci within one or a few of the examined populations; Table 2). In fact, only 1–2 loci showed substantial levels of variation (i.e. loci with at least two alleles with allele frequencies higher than 0.05) for each of the species (Table 2). Consequently, compared to those reported for other moss species (Wyatt et al., 1989b), the different measures of the amount of genetic variability within populations of these species showed on average rather low values (Table 1): P: 6.3–18.8%, A: 1.1–1.3, and HS: 0.008–0.059. Many more polymorphic loci were observed in populations of P. juniperinum and P. piliferum (Table 1). In addition to other loci showing only variation for rare alleles, 6–8 loci showed substantial levels of variation within each of these species (Table 2). As such, P. piliferum and in particular P. juniperinum showed considerable higher values for the different measures of genetic variability (Table 1): P: 14.3–57.1, A: 1.2–1.8, and HS: 0.064–0.170. A one-way ANOVA revealed that these differences in mean HS-values between the species (Table 1) were significant (P < 0.001). An additional multiple comparison of means (Dunn–Šidàk Method; Sokal & Rohlf, 1995) showed that there are, in fact, two homogeneous groups (P. commune, P. uliginosum and P. formosum vs. P. piliferum and P. juniperinum), and within these groups the means of HS are not significantly different from one another. Hence, P. piliferum and P. juniperinum show significantly higher levels of genetic variation than the other three Polytrichum species.
As there were differences in both sample size and number of loci examined between these groups, the results might be biased. However, when the ‘additional’ loci that were analysed for P. juniperinum and P. piliferum and not for the other three Polytrichum species, were omitted from the analysis, the level of gene diversity (HS) became even higher in P. juniperinum and P. piliferum (data not shown). Moreover, the highest HS-values were observed for those species with on average the smallest sample sizes.
Genetic differentiation within species
Genetic differentiation between populations of the same species was studied by means of a hierarchical analysis (Table 3). As indicated by low FST-values, relatively low levels of genetic differentiation were observed between populations of any species, except P. juniperinum. At most 10% of the total variation observed could be attributed to differences between populations (Table 3), which corresponds to Nm-values (number of migrants per generation) of 4.5 and higher (note that FST ≈ 1/(2Nm + 1) for haploid organisms; Wright, 1951; Whitlock & McCauley, 1999). P. juniperinum was an exception, because in this species almost 25% of the total genetic variation, Nm < 1.6, could be ascribed to differentiation between populations.
The effect of geographical region, i.e. populations within a country (on average 51 km apart) vs. populations from different countries (on average 406 km apart), on genetic differentiation was also analysed for each species. Table 3 shows that only for P. uliginosum the FCT-value was significantly different from zero, indicating that, for this species, genetic differentiation among regional populations was lower than between populations from different countries. However, the overall level of differentiation was still very low.
Genetic differentiation between species
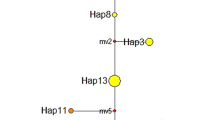
The level of divergence between different Polytrichum species was very large (Table 4, Fig. 1). Averaged over all pairwise comparisons for all species the genetic identity was only I=0.222, but it varied substantially for each of the pairwise comparisons. The identity between P. commune and P. uliginosum was significantly higher (I=0.611) than for any other pair of Polytrichum species (I=0.071–0.345). However, even for these species genetic identities were still very much lower than the genetic identities observed for populations within any of the species (range I=0.942–0.999; Table 4). This shows that the genetic divergence between species is of a different order of magnitude than at the population level.
Consensus UPGMA tree expressing overall levels of Nei’s (1987) unbiased genetic identity between 29 populations and five species of the moss genus Polytrichum (CO, P. commune; UL, P. uliginosum; FO, P. formosum; JU, P. juniperinum; PI, P. piliferum; population numbers correspond to numbers in Table 1). This analysis is based on the 15 loci that were analysed for all species. Numbers in the tree denote bootstrap values (percentage) after 1000 resamplings.
As indicated by high bootstrap values, the topology of the UPGMA tree constructed for the Polytrichum species is quite robust (Fig. 1). The species P. commune and P. uliginosum nearly always cluster together, indicating that they are much more related to each other than to any of the other Polytrichum species. Although less marked, P. juniperinum and P. piliferum also seem more related to each other than to any of the other species.
Discussion
In general, we observed relatively low levels of genetic variation, mean gene diversity (HS) ranging from 0.023 to 0.127, within populations for each of the five Polytrichum species (Table 1). The level of HS was particularly low for the species P. commune, P. uliginosum and P. formosum. These values of gene diversity are considerably lower than those observed for vascular plants (Hamrick & Godt, 1990) and also lower than those observed for populations of most other moss genera (De Vries et al., 1983, 1989; Wyatt et al., 1989a; Cronberg et al., 1997; Cronberg, 1998), although such exceptionally low levels have also been reported for some other moss species (Hofman, 1991; Wyatt, 1992). On the other hand, we observed significant differences in the amount of genetic variability among the Polytrichum species: P. piliferum and in particular P. juniperinum showed 3–5 times significantly higher values of HS than the other three examined species. Within other moss genera also considerable ranges in HS-values have been observed (De Vries et al., 1983, 1989; Hofman, 1991; Wyatt, 1992; Cronberg, 1998), which have been accounted for by differences in ecology and/or life history of the species involved. Both Hofman (1991) and Cronberg (1998), although from a different perspective, suggested such differences in genetic variation to be the consequence of differences in breeding system: monoecy vs. dioecy. Because all Polytrichum species studied here are dioecious, this can not explain the differences in the level of genetic variability we observed for these species. Although it is not impossible that the observed differences in the amount of genetic variation between Polytrichum species might be the result of historical events, we rather attribute them to differences in habitat and life history. Polytrichum commune, and in particular P. uliginosum and P. formosum are often found in more stable habitats, like peat bogs and forests (Touw & Rubers, 1989), where clonal growth might outweigh sexual reproduction and recruitment of new individuals (genets), whereas P. piliferum and P. juniperinum are more often observed in exposed and dynamic, sandy habitats (Touw & Rubers, 1989), where turnover of genets is probably much more rapid, and, as a consequence, sexual reproduction might be more important. Predominance of asexual reproduction in P. commune, P. uliginosum and P. formosum, and predominance of sexual reproduction in P. piliferum and P. juniperinum therefore would account for the higher level of gene diversity in the last two species. A comparable situation has been observed for the perennial grass Agrostis stolonifera (Kik et al., 1992), where in stable meadow habitats a low number of large clones and a low level of genotypic diversity were found, whereas in dynamic habitats, such as sand dunes, there were many genets and genotypic diversity was high.
In general, differentiation between populations within species was observed to be low and, except for P. juniperinum, FST-values were 0.10 or less (Table 3). These FST-values corresponds to Nm-values greater than 4 (Wright, 1951; Whitlock & McCauley, 1999), indicating sufficient gene flow, probably by effective spore dispersal, to prevent substantial genetic differentiation between populations. The fact that within most of the species there appears to be hardly any differentiation between Danish and Dutch populations indicates that spores probably easily disperse over large distances, which agrees well with Van Zanten & Pócs (1981), who showed that spores can disperse over large distances. However, some caution is needed, because the number of polymorphic loci, upon which FST-values were based, is low, especially in P. commune, P. uliginosum and P. formosum. On the other hand, the level of genetic differentiation within P. piliferum, based on six substantially more polymorphic loci, is not different from that in P. uliginosum and P. formosum. Moreover, preliminary results using much more variable microsatellite loci, revealed, compared to allozymes, similar FST-values for P. formosum.
Compared to the other Polytrichum species, P. juniperinum shows much higher levels of genetic differentiation between populations. Under the assumption that, for bryophytes, allozyme variation is selectively neutral in most cases (Stenøien, 1999), this indicates that gene flow is reduced in P. juniperinum (Table 3). As spores from P. juniperinum have more or less the same size as those from the other Polytrichum species (Touw & Rubers, 1989), spore size differences are unlikely to explain this observed difference. At the moment we do not have a satisfactory explanation for this higher level of genetic differentiation within P. juniperinum.
In a recent review on the population biology of the Polytrichaceae, Wyatt & Derda (1997) have summarized data on genetic variability for a number of Polytrichum species as observed for North American populations. They also observed relatively low levels of gene diversity for the species studied in this paper, though their results differ from ours in some respects. They observed considerably higher gene diversity values (HS) for P. commune and P. formosum, while, on the contrary, their HS-values for P. piliferum and P. juniperinum seem much lower than we observed for these two species. In part, the differences can possibly be explained by the fact that they sampled mainly diploid P. formosum populations, while for P. commune, they apparently did not distinguish between the different taxa recognized within P. commune s.l. (see Derda & Wyatt, 1990), which may have considerably inflated their estimates for these two species. For P. juniperinum and P. piliferum the differences are more puzzling, the more so because their FST-values for these species, 0.445 and 0.699, respectively (Wyatt & Derda, 1997), are considerably higher than we observed (Table 3). At the moment we do not have a satisfactory explanation for these differences, but possibly they may reflect differences between the continents in recolonization patterns after the latest glacial period.
In contrast to low levels of genetic differentiation within species, we observed substantial levels of divergence between species (Table 4; Fig. 1). Between pairs of Polytrichum species genetic identities ranged from I=0.071 to I=0.611 with an average of I=0.222, a value significantly lower than an average genetic identity of I=0.79 observed for 17 congeneric species of different genera of flowering plants (Crawford, 1983). This agrees well with the observation that, in general, mean genetic identities between congeneric moss species are lower than for congeneric species of vascular plants (Wyatt et al., 1989b). Although P. commune and P. uliginosum show a significantly higher level of relatedness than any other pair of Polytrichum species (I=0.611), it is still comparable to the range of genetic identities (I=0.51–0.63) reported for congeneric species in other moss genera (De Vries et al., 1983; Cronberg, 1998). The high level of divergence between P. formosum and the other examined Polytrichum species appears to support a division of the genus Polytrichum s.l. into Polytrichum s.s. and Polytrichastrum, which includes P. formosum and among others a number of allodiploid species (Smith, 1971; Derda & Wyatt, 2000). However, the level of divergence between the species pairs P. commune/P. uliginosum and P. piliferum/P. juniperinum is nearly of the same order of magnitude as that observed between P. formosum and each of these two species pairs, suggesting that if P. formosum is assigned to a separate genus Polytrichastrum then the species pairs P. commune/P. uliginosum and P. piliferum/P. juniperinum should also be assigned to different genera.
The high level of genetic divergence between congeneric Polytrichum species, and in fact between most congeneric species of other bryophyte genera, suggests that the evolutionary rate per se is not constrained, but rather that morphological characters evolved at a much slower rate than allozymes in bryophytes. A comparable situation has been observed for horseshoe crabs, which show striking morphological conservatism for the last 150 million years, but show as much genetic divergence at the DNA-level as other crab genera (Avise et al., 1994), indicating that the low rate of morphological change in horseshoe crabs is not due to a lack of genetic variability. Similarly, we infer, that the relative morphological stasis within the genus Polytrichum is not the consequence of lack of genetic variability. In addition, the considerably lower levels of genetic identity observed between most Polytrichum species, compared to other congeneric moss species, indicate that this genus is indeed relatively ancient, as suggested by Crum (1976) based on morphological comparisons of extant and fossil species.
In conclusion, contrasting the high levels of divergence between the Polytrichum species, we observed mostly low levels of genetic differentiation among populations within species, indicating a considerable degree of gene flow by wind-dispersed spores. On average low levels of gene diversity were observed within species, showing that these haploid moss species are indeed somewhat depauperate of genetic variation.
References
Avise, J. C., Nelson, W. S. and Sugita, H. (1994). A speciational history of ‘living fossils’: molecular evolutionary patterns in horseshoe crabs. Evolution, 48: 1986–2001.
Bijlsma, R., van Der Velde, M., Boerema, A. C., van de Zande, L. and van Zanten, B. O. (2000). Molecular techniques reveal cryptic species within Polytrichum commune (common hair-cap moss). Plant Biol, 2: 408–414.
Crawford, D. J. (1983). Phylogenetic and systematic inferences from electrophoretic studies. In: Tanksley, S. D. and Orton, T. J. (eds) Isozymes in Plant Genetics and Breeding, part A, pp. 257–287. Elsevier Science Publishers, Inc., New York.
Cronberg, N. (1998). Population structure and interspecific differentiation of the peat moss sister species Sphagnum rubellum and S. capillifolium (Sphagnaceae) in northern Europe. Plant Syst Evol, 209: 139–158.
Cronberg, N., Molau, U. and Sonesson, M. (1997). Genetic variation in the clonal bryophyte Hylocomium splendens at hierarchical geographical scales in Scandinavia. Heredity, 78: 293–301.
Crum, H. A. (1976). Mosses of the Great Lakes Forest. University Herbarium, University of Michigan, Ann Arbor.
Crum, H. A. and Anderson, L. E. (1981). Mosses of Eastern North America, 2 vols. Columbia University Press, New York.
de Vries, A., van Zanten, B. O. and van Dijk, H. (1983). Genetic variation within and between populations of two species of Racopilum (Racopilaceae, Bryopsida). Lindbergia, 9: 73–80.
de Vries, A., Bramer, J. P. J., van Zanten, B. O., Hofman, A. and Bijlsma, R. (1989). Allozyme variation in four Racopilum species including the polyploid R. tomentosum. Lindbergia, 15: 47–49.
Derda, G. S. and Wyatt, R. (1990). Genetic variation in the common hair-cap moss, Polytrichum commune. Syst Bot, 15: 592–605.
Derda, G. S. and Wyatt, R. (2000). Isozyme evidence regarding the origins of three allodiploid species of Polytrichastrum (Polytrichaceae, Bryophyta). Plant Syst Evol, 220: 37–53.
During, H. J. (1990). Clonal growth patterns among bryophytes. In: Van Groenendael, J. and De Kroon, H. (eds) Clonal Growth in Plants: Regulation and Function, pp. 153–176. SPB Academic Publishing, The Hague.
During, H. J. and van Tooren, B. F. (1987). Recent developments in bryophyte population biology. Trends Ecol Evol, 2: 89–93.
Ennos, R. A. (1990). Population genetics of bryophytes. Trends Ecol Evol, 5: 38–39.
Fritsch, R. (1982). Index to plant chromosome numbers — Bryophyta. Regn Veg, 108: 1–268.
Hamrick, J. L. and Godt, M. J. W. (1990). Allozyme diversity in plants. In: Brown, A. D. H., Clegg, M. T., Kahler, A. L. and Weir, B. S. (eds) Plant Population Genetics, Breeding, and Genetic Resources, pp. 43–63. Sinauer Associates Inc., Sunderland.
Hoekstra, R. F. (1978). Natural Selection in Varying Environments. PhD Thesis, University of Groningen.
Hofman, A. (1991). Phylogeny and population genetics of the genus Plagiothecium (Bryopsida). PhD Thesis, University of Groningen.
Kik, C., Linders, T. E. and Bijlsma, R. (1992). The distribution of cytotypes in ecologically contrasting populations of the clonal perennial Agrostis stolonifera. Evol Trends Plants, 6: 93–98.
Longton, R. E. (1976). Reproductive biology and evolutionary potential in bryophytes. J Hattori Bot Lab, 41: 205–223.
Longton, R. E. (1997). Reproductive biology and life-history strategies. Adv Bryol, 6: 65–102.
Miles, C. J. and Longton, R. E. (1987). Life history of the moss Atrichum undulatum (Hedw.) P. Beauv. Symp Biol Hung, 35: 193–207.
Nei, M. (1987). Molecular Evolutionary Genetics. Columbia University Press, New York.
Schneider, S., Kueffer, J. -M., Roessli, D. and Excoffier, L. (1997). ARLEQUIN, version 1.1: a software for population genetic data analysis. Genetic and Biometry Laboratory, University of Geneva, Switzerland.
Smith, G. L. (1971). Conspectus of the genera of Polytrichaceae. Mem New York Bot Gard, 21: 1–83.
Söderström, L. and Herben, T. (1997). Dynamics of bryophyte metapopulations. Adv Bryol, 6: 205–240.
Sokal, R. R. and Rohlf, F. J. (1995). Biometry. W. H. Freeman, San Francisco.
Stenøien, H. K. (1999). Are enzyme loci selectively neutral in haploid populations of nonvascular plants? Evolution, 53: 1050–1059.
Swofford, D. L. and Selander, R. B. (1981). BIOSYS-I. A computer program for the analysis of allelic variation in genetics. User’s manual. University of Illinois, Urbana.
Touw, A. and Rubers, W. V. (1989). De Nederlandse Bladmossen. Stichting Uitgeverij van de Koninklijke Nederlandse Natuurhistorische Vereniging, Utrecht.
van Zanten, B. O. and Pócs, T. (1981). Distribution and dispersal of bryophytes. Adv Bryol, 1: 479–562.
Whitlock, M. C. and McCauley, D. E. (1999). Indirect measures of gene flow and migration: FST ≠ 1/(4Nm + 1). Heredity, 82: 117–125.
Wright, S. (1951). The genetical structure of populations. Ann Eugen, 15: 323–354.
Wyatt, R. (1992). Conservation of rare endangered bryophytes: input from population genetics. Biol Conserv, 59: 99–107.
Wyatt, R. and Derda, G. S. (1997). Population biology of the Polytrichaceae. Adv Bryol, 6: 265–296.
Wyatt, R., Odrzykoski, I. J. and Stoneburner, A. (1989a). High levels of genetic variability in the haploid moss Plagiomnium ciliare. Evolution, 43: 1085–1096.
Wyatt, R., Stoneburner, A. and Odrzykoski, I. J. (1989b). Bryophyte isozymes: systematic and evolutionary implications. In: Soltis, D. E. and Soltis, P. S. (eds) Isozymes in Plant Biology, pp. 221–240. Dioscorides Press, Portland, OR.
Acknowledgements
We thank Heinjo During and Wilke van Delden for helpful comments on the manuscript. Heinjo During is also greatly acknowledged for providing samples of two populations. The research of M.v.d.V. is supported by the Research Council for Earth and Life Sciences (grant ALW 805-36-104), which is subsidised by the Netherlands Organization for Scientific Research (NWO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Der Velde, M., Bijlsma, R. Amount and structure of intra- and interspecific genetic variation in the moss genus Polytrichum. Heredity 85, 328–337 (2000). https://doi.org/10.1046/j.1365-2540.2000.00762.x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1046/j.1365-2540.2000.00762.x