Is Fluctuating Asymmetry a Sufficient Indicator of Stress Level in Two Lizard Species (Zootoca vivipara and Lacerta agilis) from Alpine Habitats?
Abstract
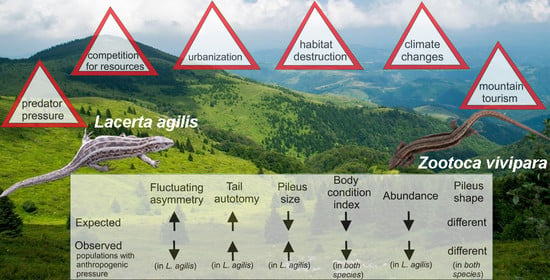
:1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Study Area
2.3. Analysis
3. Results
3.1. Differences in Pileus Size
3.2. Differences in Pileus Shape
3.3. Fluctuating Asymmetry
3.4. Body Condition Index
3.5. Tail Autotomy
3.6. Abundance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Körner, C. Mountain biodiversity, its causes and function. AMBIO J. Hum. Environ. Syst. 2004, 33, 11–17. [Google Scholar] [CrossRef]
- Nagy, L.; Grabherr, G. The Biology of Alpine Habitats; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef] [Green Version]
- Caprio, E.; Chamberlain, D.E.; Isaia, M.; Rolando, A. Landscape changes caused by high altitude ski-pistes affect bird species richness and distribution in the Alps. Biol. Conserv. 2011, 144, 2958–2967. [Google Scholar] [CrossRef]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Benito Alonso, J.L.; Coldea, G.; Dick, J.; Erschbamer, B.; Fernández Calzado, M.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Chang. 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Sato, C.F.; Wood, J.T.; Lindenmayer, D.B. The effects of winter recreation on alpine and subalpine fauna: A systematic review and meta-analysis. PLoS ONE 2013, 8, e64282. [Google Scholar] [CrossRef]
- Varricchione, M.; Di Cecco, V.; Santoianni, L.A.; Stanisci, A.; Di Febbraro, M.; Di Martino, L.; Carranza, M.L. Diagnostic species diversity pattern can provide key information on vegetation change: An insight into high mountain habitats in Central Apennines. J. Zool. Bot. Gard. 2021, 2, 453–472. [Google Scholar] [CrossRef]
- Walther, G.-R.; Beißner, S.; Burga, C.A. Trends in the upward shift of alpine plants. J. Veg. Sci. 2005, 16, 541–548. [Google Scholar] [CrossRef]
- Engler, R.; Randin, C.F.; Thuiller, W.; Dullinger, S.; Zimmermann, N.E.; Araújo, M.B.; Pearman, P.B.; Le Lay, G.; Piedallu, C.; Albert, C.H. 21st century climate change threatens mountain flora unequally across Europe. Glob. Chang. Biol. 2011, 17, 2330–2341. [Google Scholar] [CrossRef]
- Siddig, A.A.; Ellison, A.M.; Ochs, A.; Villar-Leeman, C.; Lau, M.K. How do ecologists select and use indicator species to monitor ecological change? insights from 14 years of publication in ecological indicators. Ecol. Indic. 2016, 60, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Martín, J.; López, P. An Experimental test of the costs of antipredatory refuge use in the wall lizard, Podarcis muralis. Oikos 1999, 84, 499–505. [Google Scholar] [CrossRef]
- Sato, C.F.; Wood, J.T.; Schroder, M.; Green, K.; Osborne, W.S.; Michael, D.R.; Lindenmayer, D.B. An experiment to test key hypotheses of the drivers of reptile distribution in subalpine ski resorts. J. Appl. Ecol. 2014, 51, 13–22. [Google Scholar] [CrossRef]
- Cushman, S.A. Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biol. Conserv. 2006, 128, 231–240. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Han, B.A.; Relyea, R.A.; Johnson, P.T.; Buck, J.C.; Gervasi, S.S.; Kats, L.B. The complexity of amphibian population declines: Understanding the role of cofactors in driving amphibian losses. Ann. N. Y. Acad. Sci. 2011, 1223, 108–119. [Google Scholar] [CrossRef]
- French, S.S.; Webb, A.C.; Hudson, S.B.; Virgin, E.E. Town and country reptiles: A review of reptilian responses to urbanization. Integr. Comp. Biol. 2018, 58, 948–966. [Google Scholar] [CrossRef] [Green Version]
- Lal, C.N.; Nadim, C. Factors responsible for global decline of reptilian population: A review. Intern. J. Zool. Investig. 2021, 7, 549–556. [Google Scholar]
- Amaral, M.J.; Carretero, M.A.; Bicho, R.C.; Soares, A.M.; Mann, R.M. The use of a lacertid lizard as a model for reptile ecotoxicology studies-part 1 field demographics and morphology. Chemosphere 2012, 87, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Matías-Ferrer, N.; Escalante, P. Size, body condition, and limb asymmetry in two hylid frogs at different habitat disturbance levels in Veracruz, Mexico. Herpetol. J. 2015, 25, 169–176. [Google Scholar]
- Iglesias-Carrasco, M.; Martín, J.; Cabido, C. Urban habitats can affect body size and body condition but not immune response in amphibians. Urban Ecosyst. 2017, 20, 1331–1338. [Google Scholar] [CrossRef]
- Taylor, E.N.; Diele-Viegas, L.M.; Gangloff, E.J.; Hall, J.M.; Halpern, B.; Massey, M.D.; Rödder, D.; Rollinson, N.; Spears, S.; Sun, B.; et al. The thermal ecology and physiology of reptiles and amphibians: A user’s guide. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2021, 335, 13–44. [Google Scholar] [CrossRef]
- Costantini, D.; Alonso, M.L.; Moazen, M.; Bruner, E. The relationship between cephalic scales and bones in lizards: A preliminary microtomographic survey on three lacertid species. Anat. Rec. 2010, 293, 183–194. [Google Scholar] [CrossRef]
- Kaliontzopoulou, A.; Carretero, M.A.; Llorente, G.A. Multivariate and geometric morphometrics in the analysis of sexual dimorphism variation in Podarcis lizards. J. Morphol. 2007, 268, 152–165. [Google Scholar] [CrossRef]
- Vukov, T.; Mirč, M.; Tomašević Kolarov, N.; Stamenković, S. Urbanization and the common wall lizard (Podarcis muralis) in the Pannonian basin, Serbia: Nowhere Safe? J. Zool. 2020, 310, 158–169. [Google Scholar] [CrossRef]
- Brown, W.L.; Wilson, E.O. Character displacement. Syst. Zool. 1956, 5, 49–64. [Google Scholar] [CrossRef]
- Pianka, E.R. The structure of lizard communities. Annu. Rev. Ecol. Evol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef] [Green Version]
- Stuart, Y.E.; Losos, J.B. Ecological character displacement: Glass half full or half empty? Trends Ecol. Evol. 2013, 28, 402–408. [Google Scholar] [CrossRef]
- Pfennig, D.W.; Wund, M.A.; Snell-Rood, E.C.; Cruickshank, T.; Schlichting, C.D.; Moczek, A.P. Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol. Evol. 2010, 25, 459–467. [Google Scholar] [CrossRef]
- Meiri, S.; Simberloff, D.; Dayan, T. Community-wide character displacement in the presence of clines: A test of Holarctic weasel guilds. J. Anim. Ecol. 2011, 80, 824–834. [Google Scholar] [CrossRef]
- Urošević, A.; Ljubisavljević, K.; Ivanović, A. Variation in skull size and shape of the common wall lizard (Podarcis muralis): Allometric and non-allometric shape changes. Contrib. Zool. 2014, 83, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Sottas, C.; Reif, J.; Kuczyński, L.; Reifová, R. Interspecific competition promotes habitat and morphological divergence in a secondary contact zone between two hybridizing songbirds. J. Evol. Biol. 2018, 31, 914–923. [Google Scholar] [CrossRef]
- Klingenberg, C. Analyzing fluctuating asymmetry with geometric morphometrics: Concepts, methods, and applications. Symmetry 2015, 7, 843–934. [Google Scholar] [CrossRef] [Green Version]
- Van Valen, L. A study of fluctuating asymmetry. Evolution 1962, 16, 125–142. [Google Scholar] [CrossRef]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry: Measurement, analysis, patterns. Annu. Rev. Ecol. Evol. Syst. 1986, 17, 391–421. [Google Scholar] [CrossRef]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry as a measure of developmental stability: Implications of non-normal distributions and power of statistical tests. Acta Zool. Fenn. 1992, 191, 13. [Google Scholar]
- Leary, R.F.; Allendorf, F.W. Fluctuating asymmetry as an indicator of stress: Implications for conservation biology. Trends Ecol. Evol. 1989, 4, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Benítez, H.A.; Lemic, D.; Villalobos-Leiva, A.; Bažok, R.; Órdenes-Claveria, R.; Pajač Živković, I.; Mikac, K.M. Breaking symmetry: Fluctuating asymmetry and geometric morphometrics as tools for evaluating developmental instability under diverse agroecosystems. Symmetry 2020, 12, 1789. [Google Scholar] [CrossRef]
- Vervust, B.; Van Dongen, S.; Grbac, I.; Van Damme, R. Fluctuating asymmetry, physiological performance, and stress in island populations of the Italian wall lizard (Podarcis sicula). J. Herpetol. 2008, 42, 369–377. [Google Scholar] [CrossRef]
- Băncilǎ, R.; Van Gelder, I.; Rotteveel, E.; Loman, J.; Arntzen, J.W. Fluctuating asymmetry is a function of population isolation in island lizards. J. Zool. 2010, 282, 266–275. [Google Scholar] [CrossRef]
- Lazić, M.M.; Kaliontzopoulou, A.; Carretero, M.A.; Crnobrnja-Isailović, J. Lizards from urban areas are more asymmetric: Using fluctuating asymmetry to evaluate environmental disturbance. PLoS ONE 2013, 8, e84190. [Google Scholar] [CrossRef] [Green Version]
- Mirč, M.; Kolarov, N.T.; Stamenković, S.; Vukov, T.D. Asymmetry in the common wall lizard Podarcis muralis under different levels of urbanization: The effect of trait and FA index selection. Arch. Biol. Sci. 2019, 71, 501–508. [Google Scholar] [CrossRef] [Green Version]
- Lazić, M.M.; Carretero, M.A.; Živković, U.; Crnobrnja-Isailović, J. city life has fitness costs: Reduced body condition and increased parasite load in urban common wall lizards, Podarcis muralis. Salamandra 2017, 53, 10–17. [Google Scholar]
- Forsman, A.; Lindell, L.E. Resource dependent growth and body condition dynamics in juvenile snakes: An experiment. Oecologia 1996, 108, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.M.; Warner, D.A. Body size and reproduction of a non-native lizard are enhanced in an urban environment. Biol. J. Linn. Soc. 2017, 122, 860–871. [Google Scholar] [CrossRef]
- Arnold, E.N. Caudal autotomy as a defence. In Biology of the Reptilia; Gans, C., Huey, R.B., Eds.; Alan R Liss: New York, NY, USA, 1988; pp. 235–273. [Google Scholar]
- Jaksić, F.M.; Fuentes, E.R. Correlates of tail losses in twelve species of Liolaemus lizards. J. Herpetol. 1980, 14, 137–141. [Google Scholar] [CrossRef]
- Fox, S.F.; Heger, N.A.; Delay, L.S. Social cost of tail loss in Uta stansburiana: Lizard tails as status-signalling badges. Anim. Behav. 1990, 39, 549–554. [Google Scholar] [CrossRef]
- Martin, J.; Salvador, A. Tail loss reduces mating success in the Iberian rock-lizard, Lacerta monticola. Behav. Ecol. Sociobiol. 1993, 32, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.; Avery, R.A. Effects of tail loss on the movement patterns of the lizard, Psammodromus algirus. Funct. Ecol. 1998, 12, 794–802. [Google Scholar] [CrossRef] [Green Version]
- Houlahan, J.E.; Findlay, C.S. The effects of adjacent land use on wetland amphibian species richness and community composition. Can. J. Fish. Aquat. 2003, 60, 1078–1094. [Google Scholar] [CrossRef]
- Sinsch, U.; Leskovar, C.; Drobig, A.; König, A.; Grosse, W.-R. Life-history traits in green toad (Bufo viridis) populations: Indicators of habitat quality. Can. J. Zool. 2007, 85, 665–673. [Google Scholar] [CrossRef] [Green Version]
- Soto-Rojas, C.; Suazo-Ortuño, I.; Montoya Laos, J.A.; Alvarado-Díaz, J. Habitat quality affects the incidence of morphological abnormalities in the endangered salamander Ambystoma ordinarium. PLoS ONE 2017, 12, e0183573. [Google Scholar] [CrossRef] [Green Version]
- Roitberg, E.S.; Eplanova, G.V.; Kotenko, T.I.; Amat, F.; Carretero, M.A.; Kuranova, V.N.; Bulakhova, N.A.; Zinenko, O.I.; Yakovlev, V.A. Geographic variation of life-history traits in the sand lizard, Lacerta agilis: Testing Darwin’s fecundity-advantage hypothesis. J. Evol. Biol. 2015, 28, 613–629. [Google Scholar] [CrossRef]
- Roitberg, E.S.; Orlova, V.F.; Bulakhova, N.A.; Kuranova, V.N.; Eplanova, G.V.; Zinenko, O.I.; Arribas, O.; Kratochvil, L.; Ljubisavljević, K.; Starikov, V.P.; et al. Variation in body size and sexual size dimorphism in the most widely ranging lizard: Testing the effects of reproductive mode and climate. Ecol. Evol. 2020, 10, 4531–4561. [Google Scholar] [CrossRef]
- Ryberg, K.; Olsson, M.; Wapstra, E.; Madsen, T.; Anderholm, S.; Ujvari, B. Offspring-driven local dispersal in female sand lizards (Lacerta agilis). J. Evol. Biol. 2004, 17, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Chamaillé-Jammes, S.; Massot, M.; Aragon, P.; Clobert, J. Global warming and positive fitness response in mountain populations of common lizards Lacerta vivipara. Glob. Chang. Biol. 2006, 12, 392–402. [Google Scholar] [CrossRef]
- Mullin, S.K.; Taylor, P.J. The effects of parallax on geometric morphometric data. Comput. Biol. Med. 2002, 32, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. Morphometrics and the role of the phenotype in studies of the evolution of developmental mechanisms. Gene 2002, 287, 3–10. [Google Scholar] [CrossRef]
- Rohlf, F.J. TpsDig2 Software; State University of New York: Stony Brook, NY, USA, 2017; Available online: http://www.sbmorphometrics.org/soft-dataacq.html (accessed on 12 September 2022).
- Klingenberg, C.P.; McIntyre, G.S. Geometric morphometrics of developmental instability: Analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution 1998, 52, 1363–1375. [Google Scholar] [CrossRef]
- Sheets, H.D. Integrated Morphometrics Package (IMP). Available online: https://www.animal-behaviour.de/imp/ (accessed on 21 March 2003).
- Jojić, V.; Blagojević, J.; Vujošević, M. B Chromosomes and cranial variability in yellow-necked field mice (Apodemus flavicollis). J. Mammal. 2011, 92, 396–406. [Google Scholar] [CrossRef] [Green Version]
- Fasola, E.; Biaggini, M.; Ortiz-Santaliestra, M.E.; Costa, S.; Santos, B.; Lopes, I.; Corti, C. Assessing stress response in lizards from agroecosystems with different management practices. Bull. Environ. Contam. Toxicol. 2022, 108, 196–203. [Google Scholar] [CrossRef]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Smith, G.R.; Ballinger, R.E. The ecological consequences of habitat and microhabitat use in lizards: A review. Contemp. Herpetol. 2001, 3, 1–28. [Google Scholar] [CrossRef]
- Ljubisavljevic, K.; Jovic, D.; Dzukic, G. Morphological variation of the common lizard (Zootoca vivipara Jacquin, 1787) in the central Balkans. Arch. Biol. Sci. 2010, 62, 789–796. [Google Scholar] [CrossRef]
- Molina-Borja, M.; Rodríguez-Domínguez, M.A.; González-Ortega, C.; Bohórquez-Alonso, M.L. Sexual size and shape dimorphism variation in Caesar’s lizard (Gallotia caesaris, Lacertidae) from different habitats. J. Herpetol. 2010, 44, 1–12. [Google Scholar] [CrossRef]
- Olsson, M. Male preference for large females and assortative mating for body size in the sand lizard (Lacerta agilis). Behav. Ecol. Sociobiol. 1993, 32, 337–341. [Google Scholar] [CrossRef]
- Horváthová, T.; Baláž, M.; Jandzik, D. Reproduction and morphology of the common lizard (Zootoca vivipara) from montane populations in Slovakia. Zool. Sci. 2013, 30, 92–98. [Google Scholar] [CrossRef]
- Roitberg, E.S.; Kuranova, V.N.; Bulakhova, N.A.; Orlova, V.F.; Eplanova, G.V.; Zinenko, O.I.; Shamgunova, R.R.; Hofmann, S.; Yakovlev, V.A. Variation of reproductive traits and female body size in the most widely-ranging terrestrial reptile: Testing the effects of reproductive mode, lineage, and climate. Evol. Biol. 2013, 40, 420–438. [Google Scholar] [CrossRef] [Green Version]
- Hantak, M.M.; McLean, B.S.; Li, D.; Guralnick, R.P. Mammalian body size is determined by interactions between climate, urbanization, and ecological traits. Commun. Biol. 2021, 4, 972. [Google Scholar] [CrossRef]
- Lazić, M.M.; Carretero, M.A.; Crnobrnja-Isailović, J.; Kaliontzopoulou, A. Effects of environmental disturbance on phenotypic variation: An integrated assessment of canalization, developmental stability, modularity, and allometry in lizard head shape. Am. Nat. 2015, 185, 44–58. [Google Scholar] [CrossRef] [Green Version]
- Sacchi, R.; Polo, M.S.; Coladonato, A.J.; Mangiacotti, M.; Scali, S.; Zuffi, M.A.L. The exposition to urban habitat is not enough to cause developmental instability in the common wall lizards (Podarcis muralis). Ecol. Indic. 2018, 93, 856–863. [Google Scholar] [CrossRef]
- Simbula, G.; Vignoli, L.; Carretero, M.A.; Kaliontzopoulou, A. Fluctuating asymmetry as biomarker of pesticides exposure in the Italian wall lizards (Podarcis siculus). Zoology 2021, 147, 125928. [Google Scholar] [CrossRef]
- Qualls, C.P.; Andrews, R.M. Cold climates and the evolution of viviparity in reptiles: Cold incubation temperatures produce poor-quality offspring in the lizard, Sceloporus virgatus. Biol. J. Linn. Soc. 1999, 67, 353–376. [Google Scholar]
- Ji, X.; Qiu, Q.-B.; Diong, C.-H. Influence of incubation temperature on hatching success, energy expenditure for embryonic development, and size and morphology of hatchlings in the oriental garden lizard, Calotes versicolor (Agamidae). J. Exp. Biol. 2002, 292, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Amo, L.; López, P.; Martı, J. Nature-based tourism as a form of predation risk affects body condition and health state of Podarcis muralis Lizards. Biol. Conserv. 2006, 131, 402–409. [Google Scholar] [CrossRef]
- Amo, L.; Lopez, P.; Martín, J. Habitat deterioration affects body condition of lizards: A behavioral approach with Iberolacerta cyreni lizards inhabiting ski resorts. Biol. Conserv. 2007, 135, 77–85. [Google Scholar] [CrossRef]
- Amo, L.; Lopez, P.; Martín, J. Habitat deterioration affects antipredatory behavior, body condition, and parasite load of female Psammodromus Algirus Lizards. Can. J. Zool. 2007, 85, 743–751. [Google Scholar] [CrossRef]
- Radder, R.S.; Shanbhag, B.A. Factors influencing offspring traits in the oviparous multi-clutched lizard, Calotes versicolor (Agamidae). J. Biosci. 2004, 29, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Carmona, C.A.; Castro-Arango, J.A.; Bernal-Bautista, M.H. Effect of habitat disturbance on the body condition index of the Colombian endemic lizard Anolis antonii (Squamata: Dactyloidae). S. Am. J. Herpetol. 2016, 11, 183–187. [Google Scholar] [CrossRef]
- Martín, J.; López, P. When to come out from a refuge: Risk-sensitive and state-dependent decisions in an alpine lizard. Behav. Ecol. 1999, 10, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Bateman, P.W.; Fleming, P.A. To cut a long tail short: A review of lizard caudal autotomy studies carried out over the last 20 years. J. Zool. 2009, 277, 1–14. [Google Scholar] [CrossRef]
- Cooper, W.E., Jr.; Wilson, D.S.; Smith, G.R. Sex, reproductive status, and cost of tail autotomy via decreased running speed in lizards. Ethology 2009, 115, 7–13. [Google Scholar] [CrossRef]
- Blomberg, S.P.; Shine, R. Size-based predation by kookaburras (Dacelo novaeguineae) on lizards (Eulamprus tympanum: Scincidae): What determines prey vulnerability? Behav. Ecol. Sociobiol. 2000, 48, 484–489. [Google Scholar] [CrossRef]
- Itescu, Y.; Schwarz, R.; Meiri, S.; Pafilis, P. Intraspecific competition, not predation, drives lizard tail loss on islands. J. Anim. Ecol. 2017, 86, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, A.A.; Hercus, M.J. Environmental stress as an evolutionary force. Bioscience 2000, 50, 217–226. [Google Scholar] [CrossRef]
- Laia, R.C.; Pinto, M.P.; Menezes, V.A.; Rocha, C.F.D. Asymmetry in reptiles: What do we know so far? Springer Sci. Rev. 2015, 3, 13–26. [Google Scholar] [CrossRef]
- Brooks, T.M.; Mittermeier, R.A.; Da Fonseca, G.A.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S. Global biodiversity conservation priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branton, M.; Richardson, J.S. Assessing the value of the umbrella-species concept for conservation planning with meta-analysis. Conserv. Biol. 2011, 25, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.A.M.; Rodwell, J.S.; Criado, M.G.; Gubbay, S.; Haynes, T.; Nieto, A.; Sanders, N.; Calix, M. European Red List of Habitats; Publications Office of the European Union Luxembourg: Luxembourg, 2016. [Google Scholar]




| Locality | L. agilis | Z. vivipara | Longitude, Latitude | Elevation (m asl) | Habitat Type and Vegetation | Level of Protection | Risk Factors | ||
|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | ||||||
| Kopaonik—Kukavica | 27 | 31 | 10 | 15 | 43.329° N, 20.744° E | 1606–1701 | Alpine pasture mixed with alpine shrubs | 1st degree of protection within the Kopaonik NP | No anthropogenic pressure |
| Kopaonik—Nebeske stolice | 5 | 7 | 9 | 20 | 43.262° N, 20.836° E | 1783–1877 | Alpine pasture mixed with a rocky outcrop | 2nd degree of protection within the Kopaonik NP | Heavy pressure from overcrowded tourists |
| Kopaonik—Treska | 23 | 31 | / | / | 43.260° N, 20.785° E | 1548–1594 | Alpine pasture mixed with rocky outcrops | Just outside the borders of the Kopaonik NP | No anthropogenic pressure |
| Kopaonik—Gobelja | / | / | 21 | 21 | 43.317° N, 20.823° E | 1809–1917 | Meadow mixed (low alpine shrub vegetation at the higher end mixed with spruce at the lower end) | 1st degree of protection within the Kopaonik NP | Illegal wild berry picking practices, quad biking |
| Kopaonik—Karaman greben | / | / | 21 | 33 | 43.291° N, 20.829° E | 1882–1927 | A linear habitat of alpine shrub vegetation | 3rd degree of protection within the Kopaonik NP | Large pressure (heavy machinery at multiple construction sites, skiing infrastructure), quad biking |
| Divčibare (Serbia) | 18 | 16 | / | / | 44.108° N, 19.990° E | 960 | Subalpine pasture | No protection | Popular touristic destination under considerable pressure due to urbanisation, communal waste pollution, etc. |
| Mokra Gora (Serbia) | / | / | 5 | 17 | 42.837° N, 20.361° E | 1947 | Very fragmented alpine pastures and meadows | No protection | No anthropogenic pressure |
| Bistra (North Macedonia) | 26 | 43 | / | / | 41.618° N, 20.660° E | 2026 | Alpine pasture mixed with rocky outcrops | Part of the Mavrovo NP | No anthropogenic pressure |
| (a) | |||||
| Locality | Kukavica | Nebeske stolice | Treska | Bistra | Divčibare |
| Kukavica | 0.2224 | 0.1747 | 0.0114 | 0.0001 | |
| Nebeske stolice | 0.3291 | 0.9302 | 0.9992 | 0.6655 | |
| Treska | 0.4621 | 0.9066 | 0.8736 | 0.0084 | |
| Bistra | 0.0001 | 0.9340 | 0.0292 | 0.0773 | |
| Divčibare | 0.0362 | 0.9996 | 0.5779 | 0.9324 | |
| (b) | |||||
| Locality | Gobelja | Karaman greben | Kukavica | Nebeske stolice | |
| Karaman greben | 0.3835 | ||||
| Kukavica | 0.1658 | 0.0011 | |||
| Nebeske stolice | 0.7716 | 0.9959 | 0.0165 | ||
| Mokra Gora | 0.0124 | 0.3347 | 0.0001 | 0.2788 | |
| (a) | |||||
| Locality | Kukavica | Nebeske stolice | Treska | Bistra | Divčibare |
| Kukavica | 0.035/ 0.0001 | 0.013/ 0.1010 | 0.038/ 0.0001 | 0.048/ 0.0001 | |
| Nebeske stolice | 0.015/ 0.5850 | 0.032/ 0.0130 | 0.037/ 0.0030 | 0.029/ 0.0080 | |
| Treska | 0.016/ 0.0190 | 0.018/ 0.2990 | 0.033/ 0.0001 | 0.044/ 0.0001 | |
| Bistra | 0.031/ 0.0001 | 0.033/ 0.0001 | 0.035/ 0.0001 | 0.031/ 0.0001 | |
| Divčibare | 0.031/ 0.0001 | 0.033/ 0.0001 | 0.041/ 0.0001 | 0.028/ 0.0001 | |
| (b) | |||||
| Locality | Gobelja | Karaman greben | Kukavica | Nebeske stolice | Mokra Gora |
| Gobelja | 0.014/ 0.0120 | 0.021/ 0.0030 | 0.012/ 0.5290 | 0.035/ 0.0001 | |
| Karaman greben | 0.009/ 0.5060 | 0.021/ 0.0090 | 0.017/ 0.1690 | 0.035/ 0.0010 | |
| Kukavica | 0.015/ 0.1390 | 0.015/ 0.0700 | 0.022/ 0.1120 | 0.023/ 0.1210 | |
| Nebeske stolice | 0.013/ 0.2360 | 0.009/ 0.5860 | 0.018/ 0.0630 | 0.035/ 0.0380 | |
| Mokra Gora | 0.040/ 0.0001 | 0.042/ 0.0001 | 0.047/ 0.0001 | 0.044/ 0.0001 | |
| (a) | |||||
| Locality | Kukavica | Nebeske stolice | Treska | Divčibare | Bistra |
| Kukavica | 0.9971 | 0.8852 | 0.0089 | 0.0495 | |
| Nebeske stolice | 0.9622 | 0.9115 | 0.5000 | 0.7876 | |
| Treska | 0.6434 | 0.5371 | 0.0008 | 0.0047 | |
| Divčibare | 0.0170 | 0.4928 | 0.0006 | 0.8913 | |
| Bistra | 0.1544 | 0.9624 | 0.0037 | 0.5482 | |
| (b) | |||||
| Locality | Gobelja | Kukavica | Nebeske stolice | Karaman greben | |
| Gobelja | 0.0169 | 0.4636 | 0.8226 | ||
| Kukavica | 0.0087 | 0.4860 | 0.0031 | ||
| Nebeske stolice | 0.2007 | 0.4643 | 0.1622 | ||
| Karaman greben | 0.2743 | 0.2426 | 0.9843 | ||
| Locality | L. agilis | Z. vivipara |
|---|---|---|
| Kukavica | 25.9% (15/58) | 40% (10/25) |
| Nebeske stolice | 54.5% (6/11) | 41.4% (12/29) |
| Treska | 24.1% (13/54) | |
| Gobelja | 57.1% (24/42) | |
| Karaman greben | 44.4% (24/54) | |
| Divčibare | 64.7% (22/34) | |
| Bistra | 28.9% (20/69) | |
| Mokra Gora | 50.0% (11/22) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anđelković, M.; Mirč, M.; Ajduković, M.; Cvijanović, M.; Vukov, T.; Vučić, T.; Kijanović, A.; Urošević, A. Is Fluctuating Asymmetry a Sufficient Indicator of Stress Level in Two Lizard Species (Zootoca vivipara and Lacerta agilis) from Alpine Habitats? Symmetry 2023, 15, 721. https://doi.org/10.3390/sym15030721
Anđelković M, Mirč M, Ajduković M, Cvijanović M, Vukov T, Vučić T, Kijanović A, Urošević A. Is Fluctuating Asymmetry a Sufficient Indicator of Stress Level in Two Lizard Species (Zootoca vivipara and Lacerta agilis) from Alpine Habitats? Symmetry. 2023; 15(3):721. https://doi.org/10.3390/sym15030721
Chicago/Turabian StyleAnđelković, Marko, Marko Mirč, Maja Ajduković, Milena Cvijanović, Tanja Vukov, Tijana Vučić, Ana Kijanović, and Aleksandar Urošević. 2023. "Is Fluctuating Asymmetry a Sufficient Indicator of Stress Level in Two Lizard Species (Zootoca vivipara and Lacerta agilis) from Alpine Habitats?" Symmetry 15, no. 3: 721. https://doi.org/10.3390/sym15030721