Liquid Phase and Microwave-Assisted Extractions for Multicomponent Phenolic Pattern Determination of Five Romanian Galium Species Coupled with Bioassays
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preliminary Examinations
2.2. Optimization of DLLME and UA-DLLME
2.2.1. Optimization of Extraction Medium Concentration
2.2.2. Optimization of Solid:Liquid Ratio
2.2.3. Selection of Extraction Solvent Type and Volume
2.2.4. Selection of Dispersive Solvent Volume
2.2.5. Optimization of Ultrasonication Time in UA-DLLME
2.3. Reference Method: Microwave-Assisted Extraction
2.4. Total Phenolic and Flavonoid Content, Antioxidant Capacity, and Tyrosinase Inhibitory Activity
2.4.1. Total Phenolic Content (TPC) by Spectrophotometric Assay
2.4.2. Total Flavonoid Content (TFC) by Spectrophotometric Assay
2.4.3. Antioxidant Potential Assays
2.4.4. Tyrosinase Inhibitory Activity
2.5. Quantitative Analysis of Galium Species
3. Materials and Methods
3.1. Materials
3.2. Sampling and Sample Preparation
3.3. Apparatus
3.3.1. HPLC Analysis
3.3.2. Auxiliary Equipment
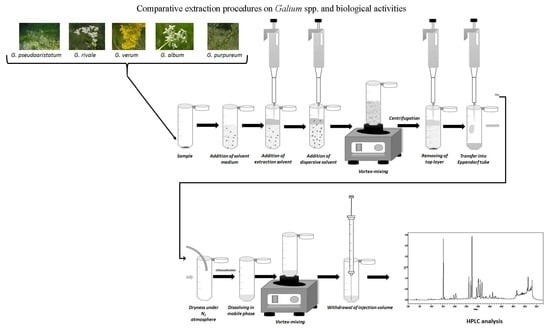
3.4. Extraction Procedures
3.4.1. DLLME and UA-DLLME
3.4.2. Salting-Out-LLE
3.4.3. Sugaring-Out-LLE
3.4.4. MAE
3.5. Total Phenolic and Flavonoid Content, Antioxidant Capacity, and Tyrosinase Inhibitory Activity
3.5.1. Antioxidant Assays
Total Phenolic Content (TPC) by Spectrophotometric Assay
Total Flavonoid Content (TFC) by Spectrophotometric Assay
DPPH Radical Scavenging Assay
Trolox Equivalent Antioxidant Capacity (TEAC) Assay
FRAP Assay
3.5.2. Tyrosinase Inhibitory Activity
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| β-CD | β-cyclodextrin |
| DLLME | Dispersive Liquid–Liquid MicroExtraction |
| DMSO | Dimethyl sulfoxide |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl-hydrate |
| d.w. | dry weight |
| FRAP | Ferric Reducing Antioxidant Power |
| GAE | Gallic Acid Equivalents |
| GC | Gas Chromatography |
| HPLC | High Performance Liquid Chromatography |
| HPLC-PDA | High Performance Liquid Chromatography-Photodiode array detector |
| ILs | ionic liquids |
| KA | Kojic Acid |
| KAE | Kojic Acid Equivalents |
| LPME | Liquid Phase MicroExtraction |
| MAE | Microwave-Assisted Extraction |
| NADES | NAtural Deep Eutectic Solvent |
| QE | Quercetin Equivalents |
| SA-LLE | Salting-out Liquid-liquid extraction |
| SULLE | Sugaring-out Liquid-liquid extraction |
| TCPC | Total Concentration of Phenolic Compounds |
| TE | Trolox Equivalents |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| TFC | Total Flavonoid Content by spectrophotometric assay |
| TPC | Total Phenolic Content by spectrophotometric assay |
| UA-DLLME | Ultrasound-Assisted Dispersive Liquid–Liquid MicroExtraction |
References
- Efferth, T. Perspectives for globalized natural medicines. Chin. J. Nat. Med. 2011, 9, 1–6. [Google Scholar] [CrossRef]
- Böjthe-Horváth, K.; Kocsis, A.; Párkány, L.; Simon, K. A new iridoid glycoside from Galium verum L. First X-ray analysis of a tricyclic iridoid glycoside. Tetrahedron Lett. 1982, 23, 965–966. [Google Scholar] [CrossRef]
- Mitova, M.I.; Anchev, M.E.; Handjieva, N.V.; Popov, S.S. Iridoid patterns in Galium L. and some phylogenetic considerations, Zeitschrift Fur Naturforsch. J. Biosci. 2002, 57, 226–234. [Google Scholar] [CrossRef]
- Demirezer, L.O.; Gürbüz, F.; Güvenalp, Z.; Ströch, K.; Zeeck, A. Iridoids, flavonoids and monoterpene glycosides from Galium verum subsp. Verum. Turk J. Chem. 2006, 30, 525–534. [Google Scholar]
- Zhao, C.C.; Shao, J.H.; Li, X.; Kang, X.D.; Zhang, Y.W.; Meng, D.L.; Li, N. Flavonoids from Galium verum L. J. Asian Nat. Prod. Res. 2008, 10, 611–615. [Google Scholar] [CrossRef]
- Zhao, C.C.; Shao, J.H.; Li, X.; Xu, J.; Wang, J.H. A new anthraquinone from Galium verum L. Nat. Prod. Res. 2006, 20, 981–984. [Google Scholar] [CrossRef]
- Lakić, N.S.; Mimica-Dukić, N.M.; Isak, J.M.; Božin, B.N. Antioxidant properties of Galium verum L. (Rubiaceae) extracts. Cent. Eur. J. Biol. 2010, 5, 331–337. [Google Scholar] [CrossRef]
- Khalili, M.; Ebrahimzadeh, M.A.; Safdari, Y. Antihaemolytic activity of thirty herbal extracts in mouse red blood cells. Arh. Hig. Rada Toksikol. 2014, 65, 399–406. [Google Scholar] [CrossRef]
- Il’ina, T.V.; Kovaleva, A.M.; Goryachaya, O.V.; Aleksandrov, A.N. Essential oil from Galium verum flowers. Chem. Nat. Compd. 2009, 45, 587–588. [Google Scholar] [CrossRef]
- Schmidt, M.; Scholz, C.; Gavril, G.; Otto, C.; Polednik, C.; Roller, J.; Hagen, R. Effect of Galium verum aqueous extract on growth, motility and gene expression in drug-sensitive and -resistant laryngeal carcinoma cell lines. Int. J. Oncol. 2014, 44, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Menković, N.; Šavikin, K.; Tasić, S.; Zdunić, G.; Stešević, D.; Milosavljević, S.; Vincek, D. Ethnobotanical study on traditional uses of wild medicinal plants in Prokletije Mountains (Montenegro). J. Ethnopharmacol. 2011, 133, 97–107. [Google Scholar] [CrossRef]
- Vlase, L.; Mocan, A.; Hanganu, D.; Gheldiu, A.; Crișan, G. Comparative study of polyphenolic content, antioxidant and antimicrobial activity of four Galium species (Rubiaceae). Dig. J. Nanomater. Biostruct. 2014, 9, 1085–1094. [Google Scholar]
- Pieroni, A.; Quave, C.L. Traditional pharmacopoeias and medicines among Albanians and Italians in southern Italy: A comparison. J. Ethnopharmacol. 2005, 101, 258–270. [Google Scholar] [CrossRef]
- Schmidt, M.; Polednik, C.; Roller, J.; Hagen, R. Galium verum aqueous extract strongly inhibits the motility of head and neck cancer cell lines and protects mucosal keratinocytes against toxic DNA damage. Oncol. Rep. 2014, 32, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; Rutkowska, M.; Owczarek, K.; Tobiszewski, M.; Namieśnik, J. Extraction with environmentally friendly solvents. TrAC. 2017, 91, 12–25. [Google Scholar] [CrossRef]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Khezeli, T.; Daneshfar, A.; Sahraei, R. A green ultrasonic-assisted liquid-liquid microextraction based on deep eutectic solvent for the HPLC-UV determination of ferulic, caffeic and cinnamic acid from olive, almond, sesame and cinnamon oil. Talanta 2016, 150, 577–585. [Google Scholar] [CrossRef]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of deep eutectic solvents in analytical chemistry. A review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Tundis, R.; Menichini, F. Natural and synthetic tyrosinase inhibitors as antibrowning agents: An update. Compr. Rev. Food Sci. Food Saf. 2012, 11, 378–398. [Google Scholar] [CrossRef]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic analyses, in vitro biological activities and cytotoxicity of Cannabis sativa L. essential oil: A multidiscipl.inary study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Di Giacomo, S.; Amatore, D.; Locatelli, M.; Vitalone, A.; Toniolo, C.; Rotino, G.L.; Lo Scalzo, R.; Palamara, A.T.; Marcocci, M.E.; et al. A Polyphenol Rich Extract from Solanum melongena L. DR2 Peel Exhibits Antioxidant Properties and Anti-Herpes Simplex Virus Type 1 Activity In Vitro. Molecules 2018, 23, 2066. [Google Scholar] [CrossRef]
- Boutaoui, N.; Zaiter, L.; Benayache, F.; Benayache, S.; Cacciagrano, F.; Cesa, S.; Secci, D.; Carradori, S.; Giusti, A.M.; Campestre, C.; et al. Atriplex mollis Desf. Aerial Parts: Extraction Procedures, Secondary Metabolites and Color Analysis. Molecules 2018, 23, 1962. [Google Scholar] [CrossRef]
- Locatelli, M.; Macchione, N.; Ferrante, C.; Chiavaroli, A.; Recinella, L.; Carradori, S.; Zengin, G.; Cesa, S.; Leporini, L.; Leone, S.; et al. Graminex pollen: Phenolic pattern, colorimetric analysis and protective effects in immortalized prostate cells (PC3) and rat prostate challenged with LPS. Molecules 2018, 23, 1145. [Google Scholar] [CrossRef] [PubMed]
- Boutaoui, N.; Zaiter, L.; Benayache, F.; Benayache, S.; Carradori, S.; Cesa, S.; Giusti, A.M.; Campestre, C.; Menghini, L.; Innosa, D.; et al. Qualitative and Quantitative Phytochemical Analysis of Different Extracts from Thymus algeriensis Aerial Parts. Molecules 2018, 23, 463. [Google Scholar] [CrossRef]
- Melucci, D.; Locatelli, M.; Locatelli, C.; Zappi, A.; De Laurentiis, F.; Carradori, S.; Campestre, C.; Leporini, L.; Zengin, G.; Picot, C.M.N.; et al. A Comparative Assessment of Biological Effects and Chemical Profile of Italian Asphodeline lutea Extracts. Molecules 2018, 23, 461. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, A.; Locatelli, M.; Kabir, A.; Furton, K.G.; Macerola, D.; Sperandio, E.; Piccolantonio, S.; Ulusoy, H.I.; Maroni, F.; Bruni, P.; et al. Comparison between Exhaustive and Equilibrium Extraction Using Different SPE Sorbents and Sol-Gel Carbowax 20M Coated FPSE Media. Molecules 2019, 24, 382. [Google Scholar] [CrossRef] [PubMed]
- Hemwimon, S.; Pavasant, P.; Shotipruk, A. Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia. Sep. Purif. Technol. 2007, 54, 44–50. [Google Scholar] [CrossRef]
- Mollica, A.; Locatelli, M.; Macedonio, G.; Carradori, S.; Sobolev, A.P.; De Salvador, R.F.; Monti, S.M.; Buonanno, M.; Zengin, G.; Angeli, A.; et al. Microwave-assisted extraction, HPLC analysis, and inhibitory effects on carbonic anhydrase I, II, VA, and VII isoforms of 14 blueberry Italian cultivars. J. Enzyme Inhib. Med. Chem. 2016, 6366, 1–6. [Google Scholar] [CrossRef]
- Hashizaki, K.; Kageyama, T.; Inoue, M.; Taguchi, H.; Ueda, H.; Saito, Y. Study on preparation and formation mechanism of n-alkanol/water emulsion using α-cyclodextrin. Chem. Pharm. Bull. 2007, 55, 1620–1625. [Google Scholar] [CrossRef]
- Inoue, M.; Hashizaki, K.; Taguchi, H.; Saito, Y. Preparation and characterization of n-alkane/water emulsion stabilized by cyclodextrin. J. Oleo. Sci. 2009, 58, 85–90. [Google Scholar] [CrossRef]
- Duchêne, D.; Bochot, A.; Yu, S.C.; Pépin, C.; Seiller, M. Cyclodextrins and emulsions. Int. J. Pharm. 2003, 266, 85–90. [Google Scholar] [CrossRef]
- Diuzheva, A.; Carradori, S.; Andruch, V.; Locatelli, M.; De Luca, E.; Tiecco, M.; Germani, R.; Menghini, L.; Nocentini, A.; Gratteri, P.; et al. Use of Innovative (Micro)Extraction Techniques to Characterise Harpagophytum procumbens Root and its Commercial Food Supplements. Phytochem. Anal. 2018, 3, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Industrial Crops & Products screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crop. Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Zengin, G.; Menghini, L.; Malatesta, L.; De Luca, E.; Bellagamba, G.; Uysal, S.; Aktumsek, A.; Locatelli, M. Comparative study of biological activities and multicomponent pattern of two wild Turkish species: Asphodeline anatolica and Potentilla speciosa. J. Enzyme Inhib. Med. Chem. 2016, 31, 203–208. [Google Scholar] [CrossRef]
- Mocan, A.; Schafberg, M.; Crisan, G.; Rohn, S. Determination of lignans and phenolic components of Schisandra chinensis (Turcz.) Baill. using HPLC-ESI-ToF-MS and HPLC-online TEAC: Contribution of individual components to overall antioxidant activity and comparison with traditional antioxidant assays. J. Funct. Food. 2016, 24, 579–594. [Google Scholar] [CrossRef]
- Mocan, A.; Crişan, G.; Vlase, L.; Crişan, O.; Vodnar, D.C.; Raita, O.; Gheldiu, A.M.; Toiu, A.; Oprean, R.; Tilea, I. Comparative studies on polyphenolic composition, antioxidant and antimicrobial activities of Schisandra chinensis leaves and fruits. Molecules 2014, 19, 15162–15179. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Dueñas, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterization of phenolic compounds and antioxidant properties of Glycyrrhiza glabra L. rhizomes and roots. RSC Adv. 2015, 5, 26991–26997. [Google Scholar] [CrossRef]
- Damiano, S.; Forino, M.; De, A.; Vitali, L.A.; Lupidi, G.; Taglialatela-Scafati, O. Antioxidant and antibiofilm activities of secondary metabolites from Ziziphus jujuba leaves used for infusion preparation. Food Chem. 2017, 230, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Likhitwitayawuid, K.; Sritularak, B. A new dimeric stilbene with tyrosinase inhibitory activity from Artocarpus gomezianus. J. Nat. Prod. 2001, 64, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



| TPC (mg GAE/g Extract) | TFC (mg QE/g Extract) | DPPH Scavenging (mg TE/g Extract) | ABTS Scavenging (mg TE/g Extract) | FRAP (mg TE/g Extract) | Tyrosinase Inhibit. (mg KAE/g Extract) | Tyrosinase Inhibit. (% Inhibition) | |
|---|---|---|---|---|---|---|---|
| G. verum | 3.1 ± 0.3 a,e | 8.60 ± 0.07 a,e | 1.9 ± 0.5 a,e | 6.15 ± 0.02 a,e | 21.9 ± 0.9 a,c,e | 7.3 ± 0.2 a,e | 36.96 |
| G. album | 2.7 ± 0.1 a,e | 4.88 ± 0.03 a,e | 1.1 ± 0.2 a,e | 6.1 ± 0.1 a,e | 17.19 ± 0.04 b,e | 13.8 ± 3.4 e | 70.98 |
| G. rivale | 1.3 ± 0.2 a,d,e | 4.01 ± 0.08 a,c,e | 0.4 ± 0.25 a,e | 4.5 ± 0.5 a,d,e | 12.6 ± 0.2 a,e | na | na |
| G. pseudoaristatum | 4.5 ± 0.6 a,d,e | 6.7 ± 0.3 a,c | 1.9 ± 0.1 a,e | 7.6 ± 0.2 a,d,e | 19.4 ± 0.9 a,b,c,e | 2.5 ± 1.6 e | 4.66 |
| G. purpureum | 10.3 ± 0.8 e | 8.50 ± 0.04 a,e | 6.3 ± 0.7 e | 16.7 ± 0.8 e | 45.2 ± 1.1 e | 6.3 ± 1.4 a,e | 29.71 |
| KA (0.1 mg/mL) | 62.52 |
| Metabolite | Galium Species | ||||
|---|---|---|---|---|---|
| G. verum | G. album | G. rivale | G. pseudoaristatum | G. purpureum | |
| Conc., μg/g | |||||
| Mean (±S.D.) | Mean (±S.D.) | Mean (±S.D.) | Mean (±S.D.) | Mean (±S.D.) | |
| Gallic acid | 109 (±7) a,b,e | 23.2 (±1.5) a,b,e | |||
| Catechin | 380 (±96) a,e | 63.5 (±2.5) a,e | 203 (±30) a,e | ||
| Chlorogenic acid | 2986 (±75) e | 8310 (±231) e | 10192 (±34) e | 1640 (±30) e | 5572 (±205) e |
| 3-OH benzoic acid | 853 (±184) e | 87.4 (±12.6) a,c | 374 (±16) b,c,d,e | ||
| Rutin | 3624 (±97) e | 275 (±38) a,c,e | 987 (±24) e | 283 (±5) a,b,c,d,e | 137 (±13) a,d,e |
| Sinapinic acid | 55.7 (±0.9) a,d,e | 203 (±81) a,b | |||
| Quercetin | 89.6 (±15.5) a,e | 84.1 (±8.3) a,c,e | 67.9 (±11.6) a,c,e | ||
| Carvacrol | 101 (±2) a,e | 84.1 (±0.9) a,c,e | 102.6 (±0.9) a,e | 81.8 (±2.1) a,c,e | 162 (±26) a,c,e |
| Total μg/g | 7654 (±222) | 9133 (±253) | 11345 (±42) | 2526 (±47) | 6471 (±223) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocan, A.; Diuzheva, A.; Bădărău, S.; Moldovan, C.; Andruch, V.; Carradori, S.; Campestre, C.; Tartaglia, A.; De Simone, M.; Vodnar, D.; et al. Liquid Phase and Microwave-Assisted Extractions for Multicomponent Phenolic Pattern Determination of Five Romanian Galium Species Coupled with Bioassays. Molecules 2019, 24, 1226. https://doi.org/10.3390/molecules24071226
Mocan A, Diuzheva A, Bădărău S, Moldovan C, Andruch V, Carradori S, Campestre C, Tartaglia A, De Simone M, Vodnar D, et al. Liquid Phase and Microwave-Assisted Extractions for Multicomponent Phenolic Pattern Determination of Five Romanian Galium Species Coupled with Bioassays. Molecules. 2019; 24(7):1226. https://doi.org/10.3390/molecules24071226
Chicago/Turabian StyleMocan, Andrei, Alina Diuzheva, Sabin Bădărău, Cadmiel Moldovan, Vasil Andruch, Simone Carradori, Cristina Campestre, Angela Tartaglia, Marta De Simone, Dan Vodnar, and et al. 2019. "Liquid Phase and Microwave-Assisted Extractions for Multicomponent Phenolic Pattern Determination of Five Romanian Galium Species Coupled with Bioassays" Molecules 24, no. 7: 1226. https://doi.org/10.3390/molecules24071226