Introduction
Tropical mountains and especially montane forests are hotspots of biodiversity. Together with other East African mountains, Mt Kilimanjaro forms one of the globally acknowledged biodiversity hotspots (Mittermeier et al. Reference Mittermeier, Robles, Hoffmann, Pilgrim, Brooks, Mittermeier, Lamoreux and da Fonseca2004). Even though only c. 10% of the extent of the original habitats is remaining (Mittermeier et al. Reference Mittermeier, Robles, Hoffmann, Pilgrim, Brooks, Mittermeier, Lamoreux and da Fonseca2004), the Eastern Afromontane biodiversity hotspot still harbours a staggering amount of diversity and endemism (Burgess et al. Reference Burgess, Butynski, Cordeiro, Doggart, Fjeldså, Howell, Kilahama, Loader, Lovett and Mbilinyi2007; Harper et al. Reference Harper, Measey, Patrick, Menegon and Vonesh2010; Mittermeier et al. Reference Mittermeier, Turner, Larsen, Brooks, Gascon, Zachos and Habel2011), including lichens and lichen-symbiotic organisms (Suija et al. Reference Suija, Kaasalainen, Kirika and Rikkinen2018; Kaasalainen et al. Reference Kaasalainen, Tuovinen, Kirika, Mollel, Hemp and Rikkinen2021a, Reference Kaasalainen, Tuovinen, Mwachala, Pellikka and Rikkinenb; Kantelinen et al. Reference Kantelinen, Hyvärinen, Kirika and Myllys2021).
Peltigera is perhaps among the most studied of lichen genera (Miadlikowska & Lutzoni Reference Miadlikowska and Lutzoni2000; Miadlikowska et al. Reference Miadlikowska, Lutzoni, Goward, Zoller and Posada2003; Sérusiaux et al. Reference Sérusiaux, Goffinet, Miadlikowska and Vitikainen2009; Zúñiga et al. Reference Zúñiga, Leiva, Ramírez-Fernández, Carú, Yahr and Orlando2015; Jüriado et al. Reference Jüriado, Kaasalainen and Rikkinen2017; Magain et al. Reference Magain, Miadlikowska, Goffinet, Sérusiaux and Lutzoni2017a, Reference Magain, Miadlikowska, Mueller, Gajdeczka, Truong, Salamov, Dubchak, Grigoriev, Goffinet and Sérusiauxb, Reference Magain, Truong, Goward, Niu, Goffinet, Sérusiaux, Vitikainen, Lutzoni and Miadlikowska2018). The genus has been further divided into eight monophyletic sections, of which sections Peltigera and Polydactylon are most speciose, together containing over 100 species (Miadlikowska & Lutzoni Reference Miadlikowska and Lutzoni2000; Magain et al. Reference Magain, Miadlikowska, Goffinet, Sérusiaux and Lutzoni2017a, Reference Magain, Truong, Goward, Niu, Goffinet, Sérusiaux, Vitikainen, Lutzoni and Miadlikowska2018). The other sections, such as Horizontales, are significantly smaller with less than ten species each (Miadlikowska & Lutzoni Reference Miadlikowska and Lutzoni2000). However, as with many lichens, the worldwide sampling of Peltigera concentrates on certain non-tropical areas while, for example, tropical Africa is under-represented and only a small number of African Peltigera specimens have been included in studies using DNA data.
So far, 11 species of Peltigera have been reported from East Africa, including Ethiopia, Kenya, Tanzania and Uganda. These species include P. canina (L.) Willd., P. cichoracea Delise, P. didactyla (With.) J. R. Laundon, P. dolichorhiza (Nyl.) Nyl., P. polydactyloides Nyl., P. polydactylon (Neck.) Hoffm., P. praetextata (Flörke ex Sommerf.) Zopf, P. rufescens (Weis.) Humb., P. rufescentiformis (Gyeln.) C. W. Dodge, P. sorediifera (Nyl.) Vitik. (as P. lambinonii Goffinet) and P. ulcerata Müll. Arg.; of these, all but P. canina, P. rufescens and P. sorediifera have also been reported from Tanzania (Swinscow & Krog Reference Swinscow and Krog1988; Goffinet & Hastings Reference Goffinet and Hastings1995; Frisch & Hertel Reference Frisch and Hertel1998; Krog Reference Krog2000; Alstrup & Christensen Reference Alstrup and Christensen2006; Kirika et al. Reference Kirika, Ndiritu, Mugambi, Newton and Lumbsch2018; Kaasalainen et al. Reference Kaasalainen, Tuovinen, Mwachala, Pellikka and Rikkinen2021b). The phylogenetic analysis of genetic markers has revealed previously unknown diversity among Peltigera in other parts of the world and, for example, P. canina, P. didactyla, P. dolichorhiza, P. polydactylon and P. rufescens have been shown to include additional and partly still undescribed species (Goffinet et al. Reference Goffinet, Miadlikowska and Goward2003; Miadlikowska et al. Reference Miadlikowska, Lutzoni, Goward, Zoller and Posada2003; Sérusiaux et al. Reference Sérusiaux, Goffinet, Miadlikowska and Vitikainen2009; Magain et al. Reference Magain, Sérusiaux, Zhurbenko, Lutzoni and Miadlikowska2016, Reference Magain, Miadlikowska, Goffinet, Sérusiaux and Lutzoni2017a, Reference Magain, Miadlikowska, Mueller, Gajdeczka, Truong, Salamov, Dubchak, Grigoriev, Goffinet and Sérusiauxb, Reference Magain, Truong, Goward, Niu, Goffinet, Sérusiaux, Vitikainen, Lutzoni and Miadlikowska2018; Manoharan-Basil et al. Reference Manoharan-Basil, Miadlikowska, Goward, Andrésson and Miao2016; Jüriado et al. Reference Jüriado, Kaasalainen and Rikkinen2017). Here, we sampled Peltigera from various habitats on Mt Kilimanjaro in Tanzania, East Africa, and assessed the species identities and their distribution using DNA-based methods and morphology.
Material and Methods
Study location and sampling
The relatively young (c. 2.5 million years old; Nonnotte et al. Reference Nonnotte, Guillou, Le Gall, Benoit, Cotten and Scaillet2008) dormant volcano Mt Kilimanjaro in north-eastern Tanzania, located c. 330 km south of the equator, reaches 5895 m above sea level and c. 4900 m above the surrounding savannah. The elevational gradient of the mountain slopes supports a wide range of natural vegetation types, as well as many human-modified habitats (Lambrechts et al. Reference Lambrechts, Woodley, Hemp, Hemp and Nnyiti2002; Hemp Reference Hemp2005, Reference Hemp2006b; Rutten et al. Reference Rutten, Ensslin, Hemp and Fischer2015; Hemp & Hemp Reference Hemp and Hemp2018). The windward slopes receive considerable amounts of atmospheric moisture from the Indian Ocean and harbour several types of moist mountain forest with abundant and diverse epiphytes (Hemp Reference Hemp2006b; Kaasalainen et al. Reference Kaasalainen, Tuovinen, Kirika, Mollel, Hemp and Rikkinen2021a). For this study, 13 natural and disturbed habitat types were sampled on the moist southern and south-eastern slopes of the mountain: 1) natural savannah and 2) maize fields (800–1100 m alt.); 3) lower-montane forests, 4) traditional Chagga home gardens, 5) commercial coffee farms and 6) grasslands (1100–2000 m); 7) montane Ocotea forest and 8) selectively logged Ocotea forest (2100−2800 m); 9) upper-montane Podocarpus forest and 10) Podocarpus forest replaced by Erica excelsa forest as a result of fire (2800–3100 m); 11) subalpine Erica trimera forest and 12) fire disturbed Erica forest (3500–4000 m); 13) alpine Helichrysum heaths (4000–4600 m). Between the habitats, the mean annual temperature varies from c. 23 °C at the base of the mountain to 4 °C in the alpine zone, and night frosts occur above 2700 m (Hemp Reference Hemp2006a; Appelhans et al. Reference Appelhans, Mwangomo, Otte, Detsch, Nauss and Hemp2016). The relative humidity and precipitation are highest in the montane forest zones within the stable cloud condensation belt (mean annual precipitation over 2000–2400 mm, partly up to 3000 mm), from where the precipitation diminishes both up to the Helichrysum heath (c. 1000 mm) and down to the savannah (c. 600 mm) (Hemp Reference Hemp2006a; Appelhans et al. Reference Appelhans, Mwangomo, Otte, Detsch, Nauss and Hemp2016). The specimens were collected in 2016–2017 from a total of 65 sampling plots, including five plots in each above-mentioned habitat type. In each plot, specimens were collected from a 5 × 20 m central plot, as well as along two 50 m transects running in parallel 20 m apart on both sides of the central plot.
The lichen substrata were divided into five classes: 1) ground, 2) rock, 3) trunk, 4) canopy and 5) decaying wood. Ground includes specimens collected from the ground layer, except for specimens on rock surfaces and tree roots; rock includes specimens from rock surfaces and stones; trunk includes specimens mainly from tree trunks below the height of c. 2 m but also specimens from shrubs and from large tree roots; canopy includes specimens from freshly fallen branches and the canopy of occasional, relatively recently, fallen trees; decaying wood includes specimens from clearly decaying wood, including mainly tree trunks and stumps. Almost all specimens grew together with bryophytes.
Morphology and thin-layer chromatography
A total of 146 Peltigera specimens (see Supplementary Material Table S1, available online) from Mt Kilimanjaro were morphologically identified based on existing literature of studies from the area (Swinscow & Krog Reference Swinscow and Krog1988). Of these 91 specimens were selected for DNA extraction, PCR and sequencing, including 1–3 specimens of each species from each plot.
Thin-layer chromatography was performed with solvent G (toluene/ethyl acetate/formic acid, 139:83:8) following Orange et al. (Reference Orange, James and White2010). Since the secondary chemistry of most Peltigera species is well recorded for East Africa (Swinscow & Krog Reference Swinscow and Krog1988), the previous observations were confirmed from 1–2 specimens of each species, preferably collected from different habitats. Separate samples were obtained, from the thallus and soralia, of the sorediate species P. sorediifera and P. ulcerata.
DNA extraction and polymerase chain reaction (PCR)
A small fragment of each selected specimen was used to extract DNA using the GeneJET Genomic DNA Purification Kit (Fisher Scientific, Germany) following the manufacturer's instructions. PCR amplification of the fungal nuclear internal transcribed spacer region (nuITS: ITS1-5.8S-ITS2) was performed with the primers ITS5 and ITS4 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990) using GoTaq DNA polymerase (Promega, USA) as follows: 0.2 mM dNTPs, 0.2 μM of each primer, 0.5 mg ml−1 BSA and 0.025 U/μl polymerase in a total volume of 50 μl. The cycling conditions were: initial denaturation at 95 °C for 2 min, followed by 35 cycles of 45 s at 95 °C, 45 s at 56 °C, and 1 min at 72 °C, with a final elongation of 5 min at 72 °C. Sequencing was carried out, using the specified PCR primers, by LGC Genomics (Germany). After sequencing, the chromatograms of all DNA sequences were checked and edited using CodonCode Aligner (CodonCode Corporation, USA). The 81 Peltigera nuITS sequences generated in this study, together with three previously unpublished P. lepidophora nuITS sequences from northern and southern Finland, are deposited in the NCBI GenBank under Accession numbers MZ385613–MZ385696 and listed with specimen and collection information in Supplementary Material Table S1.
Phylogenetic analyses
The obtained sequences were aligned according to Peltigera sections Horizontales, Peltigera and Polydactylon (Miadlikowska & Lutzoni Reference Miadlikowska and Lutzoni2000) using PhyDE-1 v. 0.997 (Müller et al. Reference Müller, Müller and Quandt2010). Identical ITS variants were combined (see Supplementary Material Table S1: ITS variant). Using the alignments of Jüriado et al. (Reference Jüriado, Kaasalainen and Rikkinen2017) as starting points and utilizing existing studies (Sérusiaux et al. Reference Sérusiaux, Goffinet, Miadlikowska and Vitikainen2009; Magain et al. Reference Magain, Miadlikowska, Goffinet, Sérusiaux and Lutzoni2017a, Reference Magain, Truong, Goward, Niu, Goffinet, Sérusiaux, Vitikainen, Lutzoni and Miadlikowska2018), the newly obtained ITS variants were aligned with sequences from GenBank. Two ambiguous regions within ITS1 in section Peltigera, and one ambiguous region within ITS2 in section Polydactylon were removed, after which ITS variants identical to the remaining portion were further combined. This resulted in alignments of 661, 627 and 719 nts for Horizontales, Peltigera and Polydactylon, respectively. Phylogenetic analyses were performed using Bayesian inference and the data partitioned according to the marker regions (ITS1-5.8S-ITS2). Substitution models for the regions were selected using jModelTest2 (Posada Reference Posada2008) and Bayesian information criterion: K80, JC and K80 were selected for section Horizontales, and HKY + G, JC and HKY + G for sections Peltigera and Polydactylon. The analyses were run using MrBayes v. 3.2.3 (Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003) according to the principles described by Olsson et al. (Reference Olsson, Kaasalainen and Rikkinen2012) on the CIPRES Science Gateway (Miller et al. Reference Miller, Pfeiffer and Schwartz2010), with three runs of four chains each. The convergence of the parallel runs was checked after 107 generations and the first 25% was discarded as burn-in using Tracer v. 1.5 (Rambaut et al. Reference Rambaut, Drummond, Xie, Baele and Suchard2018); the results were visualized using TreeGraph2 v. 2.15 (Stöver & Müller Reference Stöver and Müller2010).
Results and Discussion
Most Peltigera species detected from Mt Kilimanjaro could be placed within existing taxa based on morphology and nuITS data. These include P. dolichorhiza (30 specimens), P. polydactyloides (28), P. praetextata (10), P. rufescentiformis (38), P. sorediifera (13) and P. ulcerata (7). Of these, P. sorediifera has not previously been reported from Tanzania. Additionally, P. seneca (18 specimens) is reported for the first time from Africa and a new species, P. alkalicola, is described in this study based on two collected specimens. Peltigera seems to be absent below the lower-montane forest zone and in the more heavily modified habitats (i.e. in natural savannah, maize fields, coffee plantations, grasslands and home gardens). Of the Peltigera species reported in this study, P. dolichorhiza, P. polydactyloides, P. praetextata and P. ulcerata had a relatively wide distribution reaching over at least three montane vegetation zones, while P. alkalicola, P. rufescentiformis, P. seneca and P. sorediifera were present only above c. 3500 m alt. in the subalpine and alpine vegetation zones.
nuITS and phylogenetic analyses
Of the 91 selected specimens from Mt Kilimanjaro, 81 were successfully sequenced. No more than one sequence failed from any single sampling plot, and the failed specimens were not concentrated on any specific species, sections, or habitats. Thirty-six of the 81 sequenced specimens represent five species of Peltigera section Peltigera (Fig. 1A). The five specimens of Peltigera praetextata all represent one ITS variant identical to P. praetextata sequences in GenBank originating from different parts of the world. The four Peltigera ulcerata specimens represent three different ITS variants and formed a well-supported (posterior probability PP = 1) group with P. ulcerata specimens originating from other parts of the world. All specimens morphologically belonging to the Peltigera didactyla group (i.e. sorediate and tomentose; Goffinet et al. Reference Goffinet, Miadlikowska and Goward2003) represent Peltigera sorediifera: the six sequenced specimens represent four ITS variants and formed a well-supported (PP = 1) group with P. sorediifera and P. lambinonii specimens from East Africa and Australia (Miadlikowska et al. Reference Miadlikowska, Lutzoni, Goward, Zoller and Posada2003; Magain et al. Reference Magain, Truong, Goward, Niu, Goffinet, Sérusiaux, Vitikainen, Lutzoni and Miadlikowska2018). Peltigera lambinonii was separated from P. didactyla and described as its own species from East Africa (Goffinet & Hastings Reference Goffinet and Hastings1995) but was later synonymized with P. sorediifera (Vitikainen Reference Vitikainen2008). Two specimens with identical ITS regions have a general morphological resemblance to Peltigera lepidophora but formed a well-supported (PP = 1) group distinct from that species, together with two sequences from GenBank originating from Alaska (USA; MH758352) and Ningxia (China; MH758356). Magain et al. (Reference Magain, Truong, Goward, Niu, Goffinet, Sérusiaux, Vitikainen, Lutzoni and Miadlikowska2018) acknowledged that these specimens collected from Alaska and Ningxia represent a species separate from Peltigera lepidophora s. str. (P. ‘lepidophora 2’) based on the analysis of ITS, β-tubulin, COR1b, COR3 and COR16 genetic regions, but the species has not yet been formally described. Based on our material collected from Mt Kilimanjaro, we now describe the new species as Peltigera alkalicola. The specimens from the USA and China are also tentatively presumed to represent P. alkalicola, even though differences in nine nucleotide sites exist in the ITS1 region. The 17 sequenced Peltigera rufescentiformis specimens represent ten ITS variants which formed a well-supported clade (PP = 1) in the phylogenetic analysis. Two variants, ‘rufescentiformis 11’ and ‘rufescentiformis 12’, are clearly more variable than the other ITS variants and differ in over ten nucleotide sites from the other P. rufescentiformis ITS variants. However, most of this variation is contained within the ambiguous regions of ITS1 that were excluded from the phylogenetic analysis.
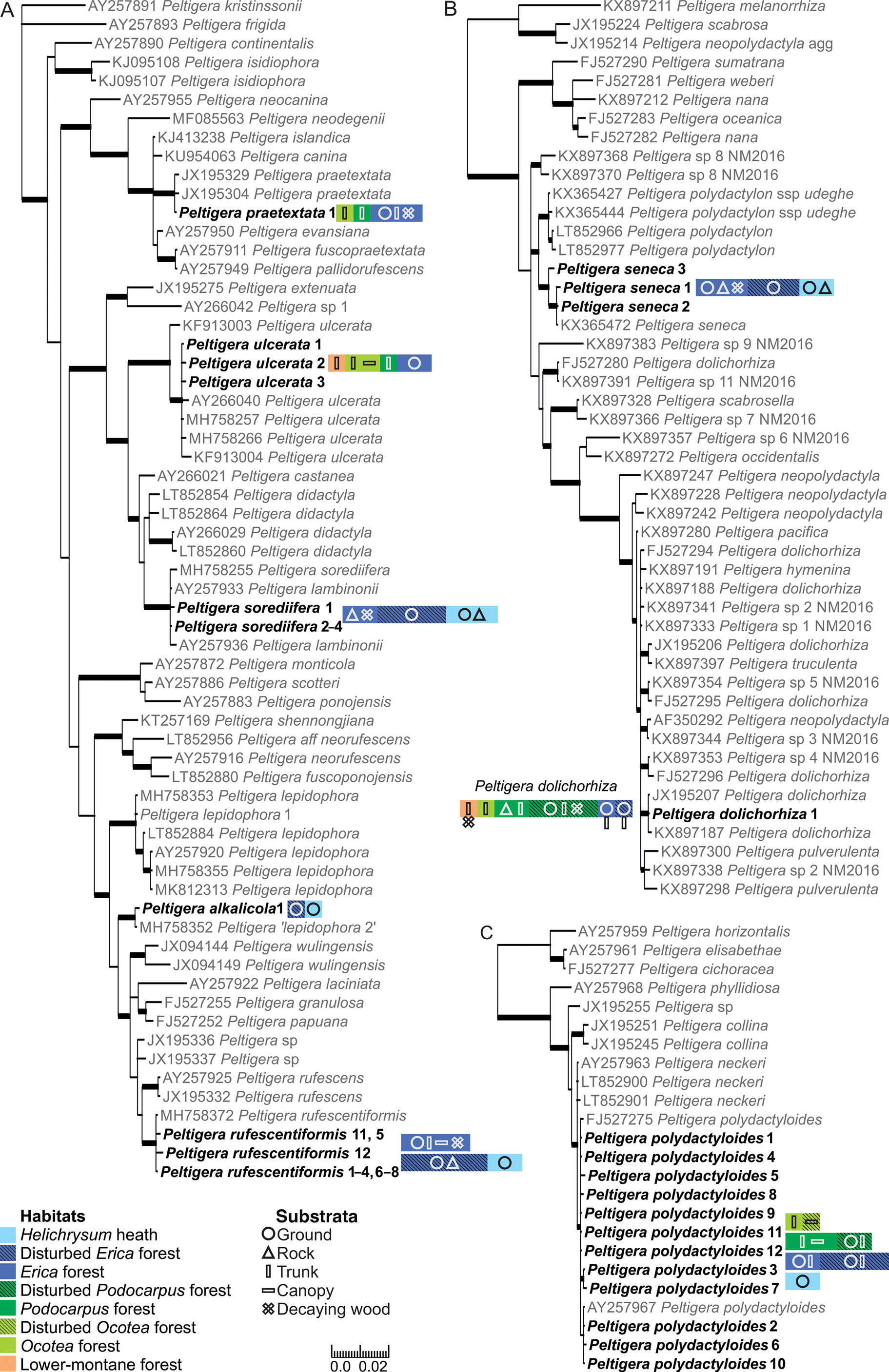
Fig. 1. Bayesian 50% majority trees based on the ITS region of the lichen mycobionts of Peltigera sections Peltigera (A), Polydactylon (B) and Horizontales (C). Specimens from Mt Kilimanjaro are shown in bold. The different ITS variants are indicated by the number directly after the species name. The colours and shapes refer to the habitats and substrata, respectively, from which each species was collected (including information from specimens with no ITS obtained), hatched colours distinguish the disturbed and natural forest habitats; one square equals a species’ presence in one sample plot. Thicker branches have a posterior probability value ≥ 0.95. Information about the sequences is available in Supplementary Material Table S1 (available online). The scale refers to nucleotide substitutions per site. In colour online.
Twenty-six sequenced specimens represent the two species of Peltigera section Polydactylon (Fig. 1B). All the specimens with a morphological resemblance to Peltigera polydactylon s. lat. belong to Peltigera seneca; the 11 sequenced specimens include three ITS variants which all formed a well-supported (PP = 1) group with P. seneca from the USA (KX365472; Magain et al. Reference Magain, Sérusiaux, Zhurbenko, Lutzoni and Miadlikowska2016). Peltigera seneca is fairly common in the high elevation habitats of Mt Kilimanjaro, Tanzania, extending the distribution of P. seneca to Africa. All 15 sequenced specimens of P. dolichorhiza represent only one ITS variant, identical to a P. dolichorhiza specimen from Kenya (JX195207; Kaasalainen et al. Reference Kaasalainen, Fewer, Jokela, Wahlsten, Sivonen and Rikkinen2013). In our analysis, the specimens formed a well-supported (PP = 1) group with a P. dolichorhiza specimen from Brazil (KX897187; Magain et al. Reference Magain, Miadlikowska, Goffinet, Sérusiaux and Lutzoni2017a). The P. dolichorhiza sequences did not form a monophyletic group in the analysis and the polyphyly of P. dolichorhiza has also been reported previously (Sérusiaux et al. Reference Sérusiaux, Goffinet, Miadlikowska and Vitikainen2009; Magain et al. Reference Magain, Miadlikowska, Mueller, Gajdeczka, Truong, Salamov, Dubchak, Grigoriev, Goffinet and Sérusiaux2017b). In the study of Magain et al. (Reference Magain, Miadlikowska, Goffinet, Sérusiaux and Lutzoni2017a), based on a worldwide sampling of Peltigera section Polydactylon, the specimen from Brazil (KX897187) was placed within P. dolichorhiza s. str. but was separate from the bulk of the P. dolichorhiza specimens which mainly originated from South and Central America, but also from Rwanda and Reunion Island in Africa and the Indian Ocean.
The only species of Peltigera section Horizontales on Mt Kilimanjaro, Peltigera polydactyloides, was represented in the dataset by 19 sequences and 12 ITS variants, which formed a well-supported (PP = 0.96) group with other P. polydactyloides specimens from East Africa downloaded from GenBank (Fig. 1C; Miadlikowska et al. Reference Miadlikowska, Lutzoni, Goward, Zoller and Posada2003; Sérusiaux et al. Reference Sérusiaux, Goffinet, Miadlikowska and Vitikainen2009).
Peltigera species on Mt Kilimanjaro
Peltigera alkalicola Kaasalainen sp. nov.
MycoBank No.: MB 840656
Similar to Peltigera lepidophora (Vain.) Bitter but differs in having smaller thalli and peltate isidia that are distinctly darker than the tomentose lamina; genetically distinct.
Type: Tanzania, Kilimanjaro Region, Kilimanjaro National Park, between Maua and Marangu routes, on soil in Helichrysum heath, 4390 m alt., −3.101144°S, 37.440764°E, 8 June 2017, U. Kaasalainen UK171254d (H—holotype). GenBank Accession no.: MZ385614.
(Fig. 2)
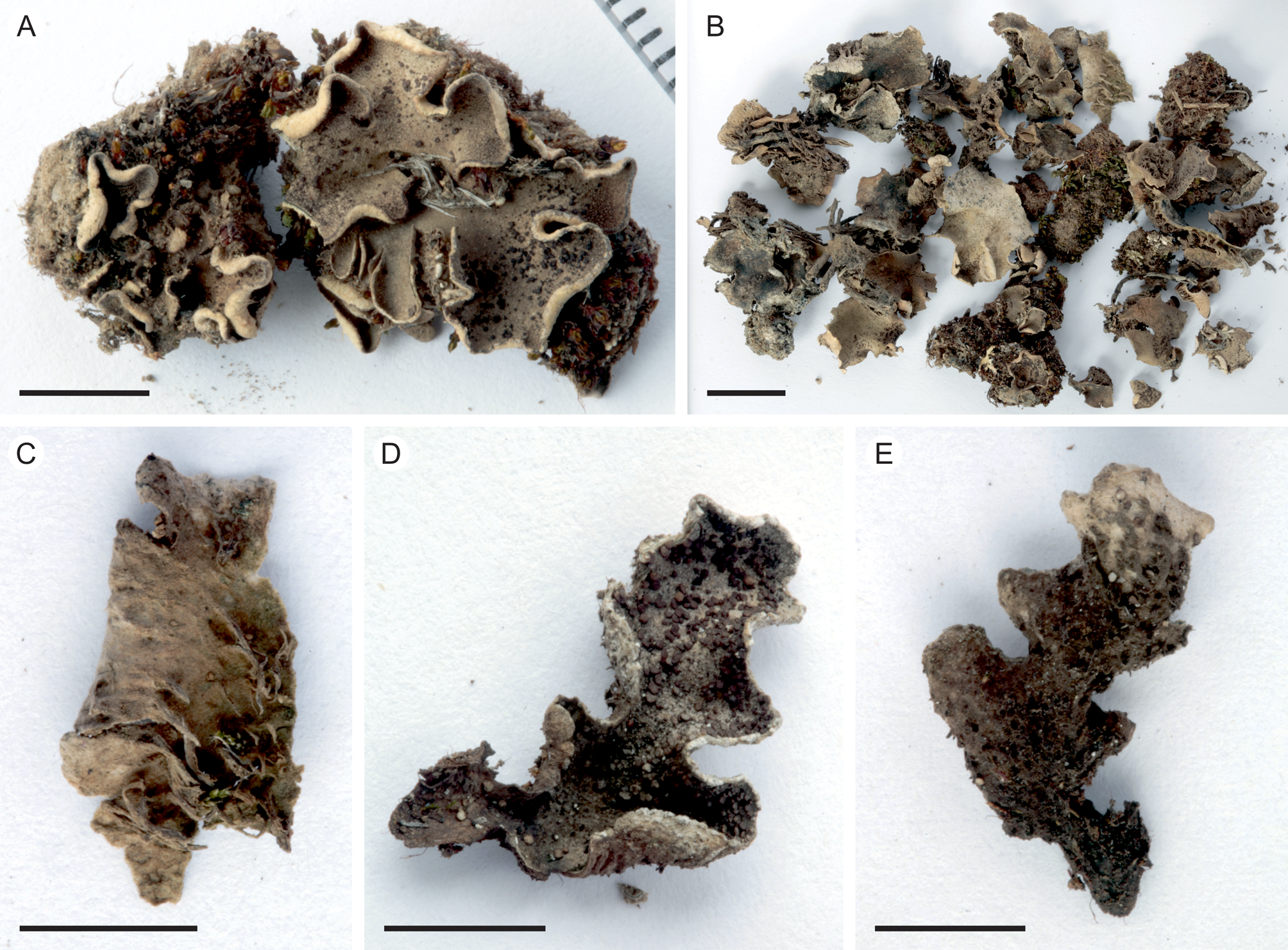
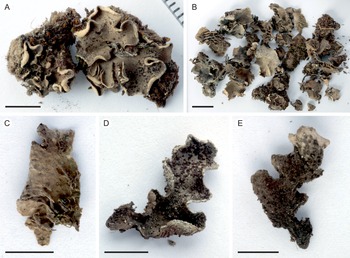
Fig. 2. Peltigera alkalicola. A, UK171254d (TYPE) from alpine Helichrysum heath, 4390 m alt. B, remainder of the same collection as the type specimen. C, lower surface and rhizines (specimen from the same collection as the type). D & E, UK171041b from disturbed Erica forest, 3940 m alt. Scales: A, C–E = 0.5 cm; B = 1 cm. In colour online.
Thallus small, up to 5 cm diam. but often smaller (c. 1–2 cm). Lobes rounded to elongate, thick, up to 1–2 cm long and 1 cm wide, with rounded ends and upturned margins, occasionally with a slightly crisped appearance. Upper surface tomentose, often slightly scabrous, greyish brown. Lower surface usually white to pale brown, with concolorous or slightly darker, low to indistinct veins. Rhizines more abundant centrally, shorter closer to the margins, simple to confluent, concolorous with the lower surface. Isidia peltate, dark brown to almost black, often clearly darker than the thallus, 0.1–0.5 mm wide, evenly distributed on the upper lamina, rarely also present on the lower side. Photobiont Nostoc.
Apothecia and pycnidia not seen.
Secondary chemistry
No secondary compounds detected by TLC.
Etymology
The specific epithet refers to the substrate preference of the species.
Ecology
Peltigera alkalicola is rare on Mt Kilimanjaro. It grows in open, high-altitude habitats including disturbed, formerly burnt subalpine Erica trimera forest and alpine Helichrysum heath at 3940–4390 m alt. on trachybasaltic lava (Downie & Wilkinson Reference Downie and Wilkinson1972). The two collection locations have 900–1100 mm of annual precipitation, 60–80% relative humidity, and an annual mean temperature of 4 °C (Appelhans et al. Reference Appelhans, Mwangomo, Otte, Detsch, Nauss and Hemp2016). Peltigera alkalicola grows on soil, occasionally with bryophytes.
Distribution
Based on data from the NCBI GenBank, Peltigera alkalicola is probably also present in Alaska, USA and Ningxia, China (Magain et al. Reference Magain, Truong, Goward, Niu, Goffinet, Sérusiaux, Vitikainen, Lutzoni and Miadlikowska2018), extending the known distribution of P. alkalicola from Africa to Asia and North America. The extreme habitats and morphological similarity to Peltigera lepidophora may have hindered previous observations of the species and P. alkalicola may be more common than so far recognized. Based on the existing observations, the distribution is expected to be limited to cold and montane regions of the world. Additionally, the observed preference for alkaline substrata may further delimit the distribution and frequency of the species.
Notes
Of the two similar species, Peltigera lepidophora and P. alkalicola, the first seems to be more common, having a distribution ranging from arctic-alpine to boreal and temperate regions. Martinez et al. (Reference Martínez, Burgaz, Vitikainen and Escudero2003) reported P. lepidophora from every continent except Africa, and DNA-confirmed observations exist at least from Northern Europe, the United Kingdom and North America (Jüriado et al. Reference Jüriado, Kaasalainen and Rikkinen2017; Magain et al. Reference Magain, Truong, Goward, Niu, Goffinet, Sérusiaux, Vitikainen, Lutzoni and Miadlikowska2018). The specimens from northern and southern Finland included in this study also represent P. lepidophora (Fig. 1A). Peltigera alkalicola and P. lepidophora also share a similar substratum ecology: P. lepidophora is reported to be terricolous on slightly calcareous (i.e. alkaline) substrata, somewhat nitrophilous, apophytic or preferring open habitats, and occasionally bryicolous (Vitikainen Reference Vitikainen1994; Jüriado et al. Reference Jüriado, Kaasalainen and Rikkinen2017); P. alkalicola specimens from Mt Kilimanjaro were terricolous in exposed habitats with only sparse vegetation, and occasionally growing on bryophytes. Additionally, the two study plots from which P. alkalicola was collected were the only sampled plots with presumably highly alkalic trachybasaltic lava as the substratum for the species.
Further specimen examined
Tanzania: Kilimanjaro Region, Kilimanjaro National Park, near Maua route, on soil with bryophytes in fire-disturbed Erica trimera forest at 3940 m alt., −3.126322°S, 37.434386°E, 2017, U. Kaasalainen UK171041b (H). GenBank Accession no.: MZ385613.
Peltigera dolichorhiza (Nyl.) Nyl.
Description
A detailed description of P. dolichorhiza in East Africa has been given by Swinscow & Krog (Reference Swinscow and Krog1988). On Mt Kilimanjaro, P. dolichorhiza can be identified based on its large (up to several dm), glabrous, relatively thin, and clearly foveate thallus with wide (up to 3.5 cm) lobes, brown, low true veins, and simple to confluent rhizines (Fig. 3A & B). It is without isidia and soredia but quite often has apothecia, especially in the Podocarpus forest zone.

Fig. 3. A & B, Peltigera dolichorhiza (UK170942a) from the lower-montane forest, 1920 m alt. C–G, morphological variation in Peltigera polydactyloides. C, UK170906e from the closed middle montane Ocotea forest, 2560 m alt. D & E, thick and glossy UK171396a from Erica forest, 3500 m alt. F & G, brown, thick, and narrow-lobed UK171080a from the open high-altitude disturbed Erica forest, 3800 m alt. Scales: A, C–E = 1 cm; B, F & G = 0.5 cm. In colour online.
Secondary chemistry
The main compounds detected by TLC from Peltigera dolichorhiza included dolichorrhizin, tenuiorin, peltidactylin, zeorin and methylgyrophorate (in the order of spot intensity). Additionally, gyrophoric acid has previously been reported from P. dolichorhiza in East Africa (Swinscow & Krog Reference Swinscow and Krog1988).
Ecology
On Mt Kilimanjaro, P. dolichorhiza can be found from the lower montane forest zone to the subalpine Erica forest zone at 1920–3520 m alt., being especially common in the Podocarpus forest zone; mostly growing on the ground and on tree trunks, occasionally on rocks or decaying wood, often with bryophytes.
Peltigera polydactyloides Nyl.
Description
A detailed description of P. polydactyloides in East Africa has been provided by Swinscow & Krog (Reference Swinscow and Krog1988). On Mt Kilimanjaro, P. polydactyloides is morphologically quite variable but is distinguished by the thick brown layer of loosely woven hyphae on the lower side, the lack of true veins, and the always glabrous and often glossy upper surface; the thallus is usually thick and the lobes up to 3 cm wide (Fig. 3D & E). Occasionally a vein-like pattern can be seen on the lower surface, especially in closed forest habitats where the thallus is also often thinner and not as glossy (Fig. 3C). Particularly in the high-altitude open habitats, P. polydactyloides may grow as a more narrow-lobed (up to 0.5–1 cm wide), very thick, and dark brown form, often with gnawed-looking margins (Fig. 3F). This may resemble Peltigera seneca but is easily identified by the thicker thallus and the thick layer of hyphae and rhizines on the lower surface (Fig. 3G). Peltigera polydactyloides also occasionally has phyllidiate margins and/or apothecia.
Secondary chemistry
The main compounds detected by TLC from Peltigera polydactyloides included zeorin, tenuiorin, and one unidentified terpenoid. Additionally, methylgyrophorate and gyrophoric acid have previously been reported from P. polydactyloides in East Africa (Swinscow & Krog Reference Swinscow and Krog1988).
Ecology
On Mt Kilimanjaro, P. polydactyloides is found from montane Ocotea forest to alpine Helichrysum heath at 2560–4190 m alt. Common especially in Podocarpus and Erica forest zones. On tree trunks and canopy branches in Ocotea and Podocarpus forest, and on soil, often with bryophytes, in Erica forest and Helichrysum heath.
Peltigera praetextata (Flörke ex Sommerf.) Zopf
Description
A detailed description of P. praetextata in East Africa has been given by Swinscow & Krog (Reference Swinscow and Krog1988). On Mt Kilimanjaro, P. praetextata can be identified based on the large thallus with wide (up to 4 cm), foveate, scarcely tomentose lobes and long, mainly simple rhizines (Fig. 4A & B). Occasionally with marginal phyllidia, rarely with apothecia.

Fig. 4. Peltigera from the upper montane Erica forest. A & B, Peltigera praetextata, with wide lobes, scarce tomentum, and simple rhizines (UK171449e). C, Peltigera rufescentiformis with thickly tomentose upper surface and wide apothecia (UK171388b). D, Peltigera rufescentiformis with dark confluent rhizines (UK171449c). E & F, Peltigera seneca with narrow, glabrous lobes and confluent rhizines (UK171426a). Scales: A = 1 cm; B–F = 0.5 cm. In colour online.
Secondary chemistry
No secondary compounds detected by TLC.
Ecology
On Mt Kilimanjaro, P. praetextata can be found from montane Ocotea forest to subalpine Erica forest at 2750–3850 m alt., but only in undisturbed forest habitats. Peltigera praetextata is most common in Erica forest but even there it is not abundant. In Ocotea and Podocarpus forest, it grows on tree trunks and climbers, in Erica forest mainly on the ground and on decaying wood, often with bryophytes.
Peltigera rufescentiformis (Gyeln.) C. W. Dodge
Description
A detailed description of P. rufescentiformis in East Africa has been given by Swinscow & Krog (Reference Swinscow and Krog1988). On Mt Kilimanjaro, P. rufescentiformis can be identified based on the relatively narrow (up to 2 cm) and smooth lobes mostly covered by thick tomentum, often with a scabrous, flaky, or stripy pattern and dark, simple to confluent rhizines (Fig. 4C & D). Often with apothecia with discs round or wider than long.
Secondary chemistry
No secondary compounds detected by TLC.
Ecology
On Mt Kilimanjaro, P. rufescentiformis is found in the subalpine Erica forest zone and alpine Helichrysum heath at 3500–4190 m alt. Common and abundant, especially in the Erica forest zone. Mainly on soil with bryophytes, often in the shelter of rocks or shrubs, occasionally on tree trunks, decaying wood or rocks.
Peltigera seneca Magain, Miadl. & Sérus.
Description
A detailed description of P. seneca is provided by Magain et al. (Reference Magain, Sérusiaux, Zhurbenko, Lutzoni and Miadlikowska2016); however, on Mt Kilimanjaro the species seems to grow slightly larger, with lobes often up to 1(–1.5) cm wide. Peltigera seneca can be distinguished from the other Peltigera species by the relatively small, glabrous, mostly smooth and often glossy thallus with narrow lobes, the dark, low veins and the dark, confluent to simple rhizines (Fig. 4E & F). Occasionally with marginal phyllidia and/or apothecia. Also P. seneca often has gnawed-looking margins similar to some P. polydactyloides specimens, but the lower surface lacks the thick layer of woolly tomentum and rhizines that identify P. polydactyloides.
Secondary chemistry
The main compounds detected by TLC from Peltigera seneca included dolichorrhizin, zeorin, tenuiorin, peltidactylin and methylgyrophorate, which is in accordance with what was described by Magain et al. (Reference Magain, Sérusiaux, Zhurbenko, Lutzoni and Miadlikowska2016).
Ecology
On Mt Kilimanjaro, P. seneca is found in subalpine Erica forest zone and alpine Helichrysum heath at 3500–4190 m alt., and it is common especially in the Erica forest zone. Growing mainly on the ground with bryophytes, often in the shelter of rocks or shrubs, occasionally on rocks or decaying wood.
Peltigera sorediifera (Nyl.) Vitik.
Description
A detailed description of P. sorediifera in East Africa is given by Goffinet & Hastings (Reference Goffinet and Hastings1995) (as Peltigera lambinonii). On Mt Kilimanjaro, P. sorediifera can be identified by the abundantly tomentose upper surface, the rounded to irregular, mainly laminal soralia often placed in thallus depressions, and the very abundant and richly branching rhizines (Fig. 5A & B). Apothecia are not seen. In more exposed or harsher conditions, P. sorediifera may resemble P. ulcerata by having small, rounded lobes without visible tomentum. However, the placement of soralia more clearly on the lamina instead of just along the margin, as well as the richly branched rhizines, identify the species. The non-sorediate specimens with thick tomentum may occasionally resemble P. rufescentiformis but the light, richly branched rhizines and occasional furry-looking lobe margins distinguish P. sorediifera.

Fig. 5. A & B, a large Peltigera sorediifera (UK171341) with tomentose upper surface, laminal soralia, and abundant, richly branched rhizines; from disturbed Erica forest, 3520 m alt. C & D, Peltigera ulcerata (UK171519c) with glabrous upper surface, rounded soralia along the margin, and mainly simple rhizines; from Ocotea forest, 2750 m alt. Scales: A & B = 1 cm; C & D = 0.5 cm. In colour online.
Secondary chemistry
Methylgyrophorate was detected from the soralia of Peltigera sorediifera. Additionally, traces of gyrophoric acid have been reported to be occasionally present in the soralia of specimens from East Africa (Goffinet & Hastings Reference Goffinet and Hastings1995).
Ecology
On Mt Kilimanjaro, P. sorediifera is relatively common in the subalpine Erica forest and rare in the alpine Helichrysum heath, at 3520–4550 m alt. Growing mainly on soil or rocks with bryophytes, occasionally on decaying wood.
Peltigera ulcerata Müll. Arg.
Description
A detailed description of Peltigera ulcerata in East Africa has been given by Swinscow & Krog (Reference Swinscow and Krog1988). On Mt Kilimanjaro, P. ulcerata can be identified based on the small, glabrous thallus with oval to round, often slightly convex soralia usually situated close to the margin of the thallus, and simple to confluent rhizines (Fig. 5C & D; but also see comments below P. sorediifera). Apothecia are not seen.
Secondary chemistry
No secondary compounds were detected by TLC from the thallus or soralia of the tested Peltigera ulcerata specimen. However, methylgyrophorate and gyrophoric acid have previously been reported from the soralia of P. ulcerata in East Africa (Swinscow & Krog Reference Swinscow and Krog1988).
Ecology
On Mt Kilimanjaro, P. ulcerata is found from lower-montane forest to subalpine Erica forest at 1920–3830 m alt., but only in undisturbed forest habitats. Peltigera ulcerata is not common in any of the studied habitats. It is epiphytic on tree trunks and canopy branches from the lower-montane to Podocarpus forest, and grows on the ground in Erica forest, occasionally with bryophytes.
Species not observed in this study
Peltigera species previously reported from East Africa but not detected in this study include P. canina, P. cichoracea, P. didactyla, P. polydactylon and P. rufescens. Of these, P. canina and P. rufescens have been reported as rare or uncommon in high montane habitats in East Africa but not from Tanzania (Swinscow & Krog Reference Swinscow and Krog1988; Frisch & Hertel Reference Frisch and Hertel1998). Whether these represent P. canina s. str. and P. rufescens s. str. or some more recently discovered morphologically similar taxa (Jüriado et al. Reference Jüriado, Kaasalainen and Rikkinen2017; Magain et al. Reference Magain, Truong, Goward, Niu, Goffinet, Sérusiaux, Vitikainen, Lutzoni and Miadlikowska2018) remains to be seen.
Peltigera cichoracea is described from Ethiopia and additionally reported as rare in the montane forests of Kenya and Tanzania in East Africa (Jatta Reference Jatta1882; Swinscow & Krog Reference Swinscow and Krog1988; Sérusiaux et al. Reference Sérusiaux, Goffinet, Miadlikowska and Vitikainen2009). Swinscow & Krog (Reference Swinscow and Krog1988) describe P. cichoracea as the ‘sorediate counterpart of P. polydactyloides’ and mention that ‘after shedding of the propagules the lobe margins take on a gnawed appearance’, a character separating it from morphologically quite similar P. polydactyloides. Some of our specimens from Mt Kilimanjaro were initially identified as P. cichoracea based on morphology and especially the gnawed appearance of the margins (e.g. Fig. 3F). However, in the phylogenetic analysis of the ITS region, all specimens fall within the same clade (Fig. 1C). Peltigera polydactyloides on Mt Kilimanjaro is both morphologically and genetically somewhat variable and further studies with additional genetic markers may reveal additional diversity, as has been the case in some other groups of Peltigera (Magain et al. Reference Magain, Miadlikowska, Mueller, Gajdeczka, Truong, Salamov, Dubchak, Grigoriev, Goffinet and Sérusiaux2017b). However, Sérusiaux et al. (Reference Sérusiaux, Goffinet, Miadlikowska and Vitikainen2009) reported P. cichoracea from Papua New Guinea based on the description of Swinscow & Krog (Reference Swinscow and Krog1988) from East Africa and, according to the phylogenetic analyses, these specimens are not closely related to P. polydactyloides (Fig. 1C; Sérusiaux et al. Reference Sérusiaux, Goffinet, Miadlikowska and Vitikainen2009). In the same study, Sérusiaux et al. (Reference Sérusiaux, Goffinet, Miadlikowska and Vitikainen2009) also reported that P. cichoracea is absent from the mountains of Rwanda (Africa) and that the type collection of the species could not be located and is presumably lost.
Peltigera didactyla s. lat. has previously been reported from Ethiopia, Kenya, Tanzania and Uganda in East Africa (Swinscow & Krog Reference Swinscow and Krog1988; Frisch & Hertel Reference Frisch and Hertel1998). However, many of these observations very probably represent P. sorediifera (Goffinet & Hastings Reference Goffinet and Hastings1995; Vitikainen Reference Vitikainen2008). Goffinet & Hastings (Reference Goffinet and Hastings1995) reported that, in East Africa, P. didactyla and P. sorediifera grow sympatrically, listing collection locations for P. sorediifera from Rwanda, Uganda and Zaire. However, they do not provide more specific information on the specimens studied or the collection details for P. didactyla s. str. in East Africa, so the exact distribution of the species in the area remains to be confirmed.
Peltigera polydactylon has previously been reported from two upper montane locations in Kenya and Tanzania (Swinscow & Krog Reference Swinscow and Krog1988). Since then, Peltigera seneca has been described as a separate species from P. polydactylon s. str. (Magain et al. Reference Magain, Sérusiaux, Zhurbenko, Lutzoni and Miadlikowska2016) and in our collections was a common species in the subalpine and alpine zones of Mt Kilimanjaro. Peltigera seneca and P. polydactylon s. str. can be distinguished chemically and genetically (Vitikainen Reference Vitikainen1994; Magain et al. Reference Magain, Sérusiaux, Zhurbenko, Lutzoni and Miadlikowska2016; Timdal & Rui Reference Timdal and Rui2021), and P. polydactylon reported by Swinscow & Krog (Reference Swinscow and Krog1988) from East Africa could represent either species. Since we found that P. seneca is a common species and did not detect P. polydactylon s. str., it is probable that previous reports of P. polydactylon from East Africa also represent P. seneca. Both species occur in North America and Northern Europe, but P. seneca has a much more restricted distribution in the temperate, hemiboreal and boreal zones (Magain et al. Reference Magain, Sérusiaux, Zhurbenko, Lutzoni and Miadlikowska2016; Timdal & Rui Reference Timdal and Rui2021).
Tropical Peltigera diversity
In conclusion, a total of eight species of Peltigera are confirmed to be present on Mt Kilimanjaro, Tanzania: P. alkalicola, P. dolichorhiza, P. polydactyloides, P. praetextata, P. rufescentiformis, P. seneca, P. sorediifera and P. ulcerata. Additionally, P. cichoracea has previously been reported from Tanzania but was not detected in this study. Peltigera polydactylon s. str. is probably not present in East Africa, and the distribution of P. didactyla s. str. remains uncertain. A summary of Peltigera in East Africa is provided in Table 1.
Table 1. Peltigera species in East Africa. Included in ‘Distribution’ are records from the present study and also observations from other studies which are listed in ‘References’. ‘Ecology’ refers to observations from this study on Mt Kilimanjaro. E = Ethiopia, K = Kenya, R = Rwanda, T = Tanzania, U = Uganda.
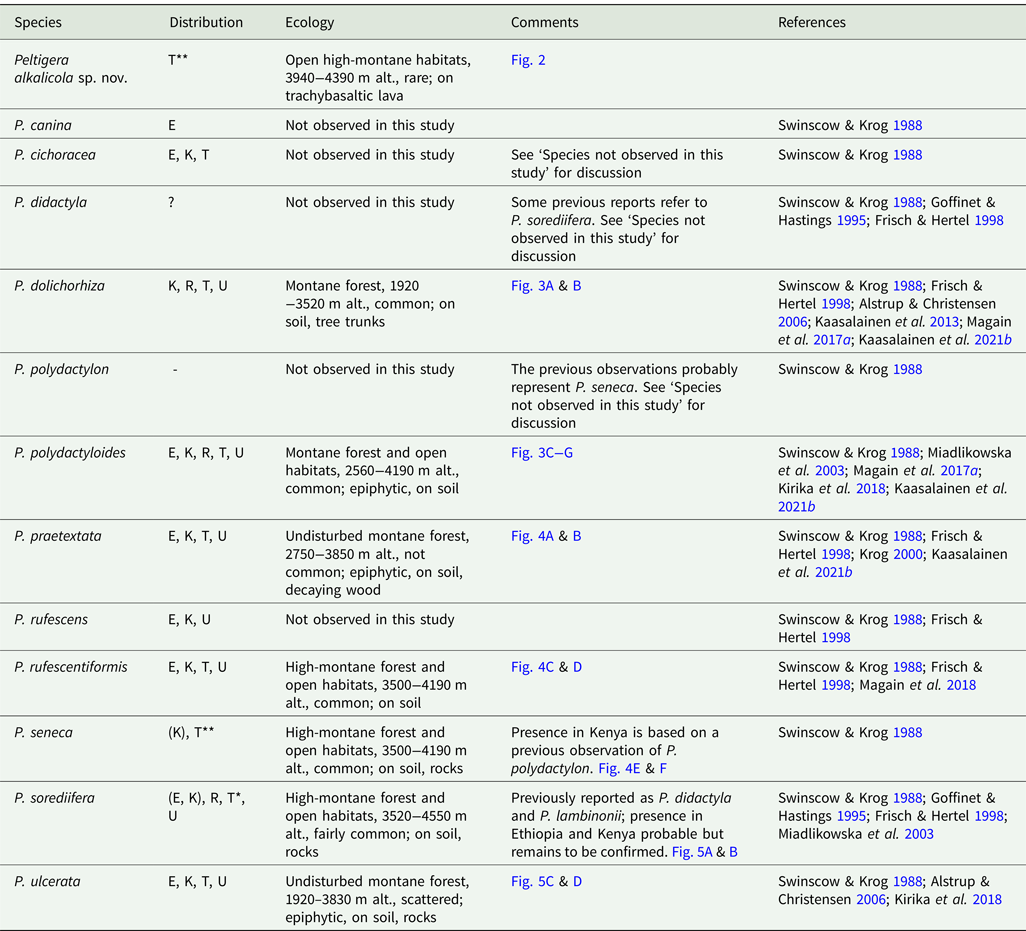
* New species for Tanzania
** New species for Tanzania and Africa
The recognized global diversity of Peltigera has increased significantly during the last few decades. New species and yet undescribed lineages have been discovered from most continents, and also many of the Peltigera species previously reported from East Africa have been divided into several species or shown to contain undescribed diversity (e.g. Miadlikowska & Lutzoni Reference Miadlikowska and Lutzoni2000; Goffinet et al. Reference Goffinet, Miadlikowska and Goward2003; O'Brien et al. Reference O'Brien, Miadlikowska and Lutzoni2009; Sérusiaux et al. Reference Sérusiaux, Goffinet, Miadlikowska and Vitikainen2009; Han et al. Reference Han, Zhang and Guo2013, Reference Han, Zhang and Guo2015, Reference Han, Xu, Yang and Guo2018, Reference Han, Yang, Bei and Guo2019; Magain et al. Reference Magain, Sérusiaux, Zhurbenko, Lutzoni and Miadlikowska2016, Reference Magain, Miadlikowska, Goffinet, Sérusiaux and Lutzoni2017a, Reference Magain, Truong, Goward, Niu, Goffinet, Sérusiaux, Vitikainen, Lutzoni and Miadlikowska2018; Manoharan-Basil et al. Reference Manoharan-Basil, Miadlikowska, Goward, Andrésson and Miao2016; Jüriado et al. Reference Jüriado, Kaasalainen and Rikkinen2017; Pardo-De la Hoz et al. Reference Pardo-De la Hoz, Magain, Lutzoni, Goward, Restrepo and Miadlikowska2018). Peltigera sections Peltigera and Polydactylon, for example, are both globally very diverse, with tens of yet undescribed species in South, Central and North America, Asia, Australasia and Europe (Jüriado et al. Reference Jüriado, Kaasalainen and Rikkinen2017; Magain et al. Reference Magain, Miadlikowska, Goffinet, Sérusiaux and Lutzoni2017a, Reference Magain, Truong, Goward, Niu, Goffinet, Sérusiaux, Vitikainen, Lutzoni and Miadlikowska2018). In the wide range of habitats sampled on Mt Kilimanjaro, however, only seven species from these sections were present and these were mostly well-known and established species. The biogeographical affinities of the Peltigera species on Mt Kilimanjaro are diverse and include: 1) widely distributed species from both hemispheres (P. praetextata and P. ulcerata); 2) disjunct species shared, for example, with South and Central America (P. dolichorhiza) and Australia (P. sorediifera); 3) African endemics (P. polydactyloides and P. rufescentiformis); 4) species from warmer (P. dolichorhiza) and colder (P. alkalicola and P. seneca) regions of the world. Even though eight Peltigera species appears low for a tropical biodiversity hotspot, the species number resembles observations from another supposed paleotropical hotspot of lichen diversity, Papua New Guinea, where likewise relatively few Peltigera species were detected (Sérusiaux et al. Reference Sérusiaux, Goffinet, Miadlikowska and Vitikainen2009). Possible reasons why our sampling from Mt Kilimanjaro could underestimate the species diversity of Peltigera in East Africa include habitat destruction and the geologically relatively young age of Mt Kilimanjaro. The extensive destruction of the extremely diverse lower-montane forest environments has impacted the observed diversity of vascular plants here (Hemp Reference Hemp2006b). The lack of natural lower altitude habitats could also explain the difference in species composition of another Peltigeralean lichen genus, Leptogium, between Mt Kilimanjaro and the nearby, much older mountains (Kaasalainen et al. Reference Kaasalainen, Tuovinen, Kirika, Mollel, Hemp and Rikkinen2021a). However, unlike Peltigera, Leptogium is very diverse in most montane habitats in the area, but especially speciose in the lower-montane forests (Kaasalainen et al. Reference Kaasalainen, Tuovinen, Kirika, Mollel, Hemp and Rikkinen2021a). Since East African Peltigera is concentrated in higher altitude habitats, the scarcity of lower-montane forest and woodlands on Mt Kilimanjaro is an unlikely cause of the observed low diversity. Additionally, in the lower-montane forest and woodland habitats of the nearby, ancient Taita Hills and Mt Kasigau, the same lower-elevation species (P. dolichorhiza, P. polydactyloides, P. praetextata and P. ulcerata) exclusively occur (U. Kaasalainen, personal observation).
Key to Peltigera species on Mt Kilimanjaro
1 Laminal peltate isidia present (rare, above c. 3500 m alt.)…………… P. alkalicola sp. nov.
Laminal peltate isidia absent………………………………………………………………. 2
2(1) Soralia present on the upper surface……………………………………………………. 3
Soralia absent…………………………………………………………………………… 4
3(2) Upper surface tomentose; rhizines abundantly branched; soralia usually not restricted to the margins (above c. 3500 m alt.) …………………………………….. P. sorediifera
Upper surface glabrous; rhizines confluent to simple; soralia restricted to the margins…..……………………………………………………………………………… P. ulcerata
4(2) Upper side entirely glabrous……………………………………………………………… 5
Upper side tomentose (occasionally just submarginally)………………………………… 7
5(4) Lower surface covered by a thick brown layer of loosely woven hyphae; true veins absent…………………………………………………..………….. P. polydactyloides
Lower side without loosely woven hyphae; with a network of distinct dark veins………. 6
6(5) Thallus small; lobes narrow (up to 1.5 cm wide), often thick and smooth (not foveate); margins may be phyllidiate (above c. 3500 m alt.)…………………………… P. seneca
Thallus usually large; lobes wide (up to 3.5 cm wide), distinctly foveate; margins usually without phyllidia/lobules…………………………………….. ……………………………………………. P. dolichorhiza
7(4) Lobes wide (up to 4 cm), often clearly foveate; tomentum on the upper surface often sparse and restricted to areas close to the margins……………………………… P. praetextata
Lobes narrow (up to 2 cm), not clearly foveate; tomentum on the upper surface thick, usually covering large portions of the thallus, tomentum often stripey, flaky or scabrous-looking (above c. 3500 m alt.)……………………………..….…. P. rufescentiformis
Acknowledgements
We are grateful to Inga Jüriado (University of Tartu) and Jouko Rikkinen (University of Helsinki) for collecting and sequencing the Peltigera lepidophora specimens from Finland. We also thank Lina Rohlmann (University of Göttingen) for supporting the laboratory work of this study as a student research assistant. This project has received funding from the German Research Foundation (DFG, grant number 408295270 to UK) and used the infrastructure and research plots of the DFG-funded research unit ‘Kilimanjaro under global change’ (KiLi).
Author Contribution
UK: conceptualization, methodology, formal analysis, investigation, data curation, writing (original draft, review and editing), visualization, supervision, project administration, funding acquisition. LB: investigation, data curation. NPM: writing (review and editing). ARS: writing (review and editing), supervision. AH: methodology, writing (review and editing).
Author ORCIDs
Ulla Kaasalainen, 0000-0001-9899-4768; Neduvoto P. Mollel, 0000-0002-4402-4667; Alexander R. Schmidt, 0000-0001-5426-4667; Andreas Hemp, 0000-0002-5369-2122.
Competing Interests
The authors declare none.
Data Accessibility
The sequences used in this study were submitted to the NCBI GenBank database under Accession numbers MZ385613–MZ385696.
Supplementary Material
To view Supplementary Material for this article, please visit https://doi.org/10.1017/S0024282922000184.