
Figure 1 Vipera aspis, photo by Joan Fuchs.
DOI: https://doi.org/10.4414/SMW.2021.w30085
From a global perspective, snakebite poses a massive burden in many parts of the world with over 100,000 dead and 400,000 maimed per annum [1]. In contrast, snakebites in Central Europe are not perceived to result in relevant morbidity. Vipers of the family Viperidae currently include 35 genera and more than 350 species worldwide, with the exception of Oceania [2]. Up to 25 species can be found in Europe, North Africa, and Asia [2]. Switzerland is home to two indigenous venomous snakes, the asp viper (Vipera aspis) and the common adder (V. berus), besides six non-venomous colubrid snakes [3]. Both vipers are small terrestrial snakes of no more than 70 cm length in total, with a short and stocky body and a subtriangular head distinct from the neck. They are of a brownish or olive colour with dark markings (figs 1 and 2). Approximately 10% of them show a melanistic appearance, turning them completely black within the first 2 years of their lives [3, 4]. They weigh a maximum of 100 g and sport solenoglyph venom fangs 3–5 mm in length, which they use for hunting and defence [3]. The species are hard to distinguish by the general population owing to their similar appearance, although the snout of V . aspis is upturned and seems more marked than that of V. berus, albeit without forming a horn covered with small scales like V. ammodytes or latastei [4]. In addition, their habitats overlap. Both snakes live in the subalpine regions of the country from 1500 to about 2300 m above sea level [3, 5]. Rarely, they can also be found below and above those altitudes. They are ovoviviparous and hibernate during the winter months. The Convention of Bern of 1979 defines catching or killing snakes in Switzerland as illegal [6]. Their venom is a mixture of proteins, enzymes and polypeptides [7]. It contains, amongst others, hyaluronidases, metalloproteinases, proteases and phospholipase A2 [8]. The effects of bites by both Viper species are very similar with mainly local symptoms, but some populations have also been shown to infrequently cause neurological and haematological problems [6–16]. The last fatal envenomation in Switzerland dates back to 1961 [7]. In the canton Valais bites by V. apsis in 99 patients over 32 years resulted in none or mild symptoms in half of the patients, moderate symptoms in 40% and severe symptoms in 10% of the patients [7]. We wanted to find out if these numbers are still pertinent.

Figure 1 Vipera aspis, photo by Joan Fuchs.

Figure 2 Vipera berus, photo courtesy of Daniel Blaser.
In Switzerland, nationwide (and complementary) consulting to the general public and doctors on poisonings and envenomation resulting from various toxins is provided by the Swiss National Poisons Information Centre (Tox Info Suisse). All calls recorded at the Swiss National Poisons Information Centre from January 1997 to December 2018 related to indigenous snakebites in Switzerland were included. Basic demographic (age, sex, region) and clinical (type of caller, circumstance of exposure) data are systematically collected for all calls related to snake bites and are standardised by a clinical toxicologist. Among patients in hospitals or medical practices for clinical care, the treating physician may provide a follow-up report. This additional information contains further details on the clinical findings, severity, causality assessment, treatment course (e.g., use of antivenom) and clinical outcomes. Standards and quality measures comprise review of each case by a senior clinical toxicologist to ensure completeness and correctness of the entered data. Exceptional cases are discussed by an internal expert panel with clinical toxicologists and poison information specialists present, and discrepancies are resolved by consensus before being entered into the database. This allows a systematic assessment of severity and causality (by Tox Info Suisse experts). The Cantonal Ethics Commission of Zurich approved the use of National Poisons Information Centre patient data without specific informed consent of the patients due to the inherent nature of poisons centre data.
Inclusion criteria were all calls related to indigenous snakebites with medical follow-up, identification of the snake by a herpetologist, and/or with compatible symptoms and circumstances of exposure when identification was not possible. Exclusion criteria comprised non-venomous snakes or lizards, exotic snakes, calls where a snake had not been observed and the symptoms did not correspond to a potential snakebite, calls from countries other than Switzerland and Liechtenstein, and calls from Swiss citizens bitten abroad. If several callers (e.g., first members of the general public, then the hospital physician, who might call several times) reported on the same episode, all of these were aggregated into one case. Follow-up was graded according to the Poisoning Severity Score [17]. Only cases with good causality, where the temporal context and the symptoms and/or circumstances of exposure were compatible with an indigenous snakebite and thus considered probable, were included in the analysis (fig. 3). Statistical analysis were performed with IBM SPSS Statistics, Version 25 (2020) and Microsoft Excel, Version 2015. We report counts and percentages (%), mean or median values as appropriate.

Figure 3 Selection of cases.
Within the study period, 1,516 calls related to reptile exposures were recorded at the Poison Centre (fig. 3). Of these, 1,364 (90%) were associated with snakes in general, 751 (50%) with indigenous snakes, and 466 (31%) with venomous indigenous snakes. Follow up was available in 243 (16%) patients, and sufficient causality was provided in 219 cases (14%) resulting in the final dataset for analysis. In 77 (35%) of these 219 patients V. aspis and in 54 (25%) V. berus were identified. In the remaining 88 (40%) identification of the species was not possible, but the clinical features and circumstances were compatible with an indigenous viper bite. Over two thirds of the patients were adults (155, 71%) and 64 (29%) patients were children. In both age groups, patients were predominantly male with 109 (70%) and 47 (73%), respectively. Table 1 provides the demographic characteristics of all patients. Only 2% of the bites occurred in an occupational setting, whereas 97% occurred during leisure activities. The majority of first calls came from hospital doctors (79%) and general practitioners (13%). Only a low proportion of direct calls were made by members of the general public (6%).
Table 1Demographics and characteristics of patients (n = 219).
Figure 4 shows the seasonal distribution of bite events, with the highest incidence predictably in the summer months, with peaks in the hot months of June (40, 18%), July (51, 23%) and August (43, 19%).
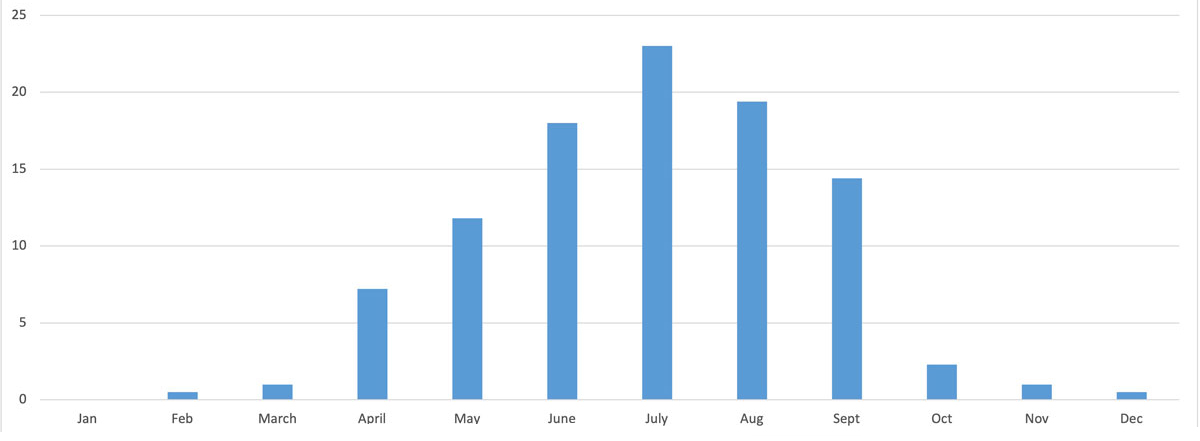
Figure 4 Seasonal distribution of indigenous venomous snakebites in Switzerland 1997–2018.
The main site of bites was the hand (72%), followed by the foot (11%) in adult patients, with comparable results (65% and 19%) in paediatric patients (table 2). Of 109 adult males, 89 (81.6 %) were bitten in the hand, whereas of the 46 adult women only half sustained a bite in the hand (23; 50 %). Of the 47 boys, 34 (72.3%) were bitten in the hand, whereas of the 17 girls only 8 (47 %) sustained a bite in the hand. Only a small proportion of the patients (8%) remained asymptomatic, with most patients exhibiting mild (43%) or moderate (36%) symptoms. Severe symptoms occurred in 13% of the patients. No fatalities were observed during the study period.
Table 2Clinical characteristics, antivenom administration and duration of hospitalisation of patients (n = 219).
1 The patient received 1 Vial (4 ml) ViperaTAb®. A full dose of antivenom consists of either 2 vials of ViperaTAb® (8 ml) or 1 vial Viperfav® (4 ml).
Available antivenoms: ViperaTAb® (MicroPharm, Great Britain), an ovine antivenom manufactured with the venom of V. berus, Viperfav® (Sanofi Pasteur MSD France), an equine antivenom manufactured with the venoms of V. aspis, berus and ammodytes, and the European Viper Venom Antitoxin (Institute of Immunology Inc, Zagreb, Croatia), an equine antivenom made with V. berus and ammodytes.
Table 3 gives an overview of symptoms and other effects experienced by the patients.
Table 3The 625 symptoms reported in 219 patients after bites by indigenous snakes (percentages calculated from 219 patients).
| Symptoms | Severity | ||
| Mild, n (%) | Moderate, n (%) | Severe, n (%) | |
| Local effects (n = 339) | |||
| Pain | 60 (27.4) | 5 (2.3) | |
| Swelling | 100 (45.6) | 56 (25.6) | 17 (7.8) |
| Paraesthesia | 12 (5.5) | ||
| Redness | 51 (23.3) | ||
| Necrosis1 | 2 (0.9) | ||
| Skin, others2 | 25 (11.4) | 10 (4.6) | 1 (0.5) |
| Muscle (n = 11) | |||
| Rhabdomyolysis3 | 7 (3.2) | 1 (0.5) | |
| Compartment syndrome | 3 (1.4) | ||
| Pulmonary (n = 15) | |||
| Oxygen saturation4 | 2 (0.9) | 3 (1.4) | 1 (0.5) |
| Dyspnoea | 8 (3.6) | 1 (0.5) | |
| Gastrointestinal (n = 114) | |||
| Nausea | 31 (14.2) | ||
| Vomiting | 33 (15.1) | 14 (6.4) | |
| Bellyache | 22 (10) | 4 (1.8) | |
| Diarrhoea | 2 (0.9) | 8 (3.6) | |
| Neurological (n = 41) | |||
| Somnolence | 11 (5) | ||
| Coma5 | 1 (0.5) | ||
| Confusion | 1 (0.5) | ||
| Vertigo | 13 (5.9) | ||
| Visual impairment | 5 (2.3) | ||
| Speech disorder | 1 (0.5) | ||
| Agitation | 3 (1.4) | 2 (0.9) | |
| Stupor | 1 (0.5) | ||
| Paralysis6 | 1 (0.5) | ||
| Hyperreflexia | 2 (0.9) | ||
| Cardiovascular (n = 59) | |||
| Hypertension7 | 3 (1.4) | 1 (0.5) | |
| Hypotension8 | 6 (2.7) | 14 (6.4) | |
| Tachycardia9 | 13 (5.9) | 3 (1.4) | |
| Bradycardia10 | 5 (2.3) | 1 (0.5) | |
| Syncope | 1 (0.5) | ||
| Shock | 6 (2.7) | ||
| ECG11 | 1 (0.5) | ||
| Cardiovascular, other12 | 2 (0.9) | 3 (1.4) | |
| Haematological (n = 38) | |||
| Quick/INR13 | 3 (1.4) | 8 (3.6) | 1 (0.5) |
| Thrombocytopenia14 | 3 (1.4) | 2 (0.9) | |
| Leucocytosis15 | 2 (0.9) | 8 (3.6) | |
| Haemolysis | 1 (0.5) | 1 (0.5) | |
| Anaemia | 2 (0.9) | ||
| Hypopotassaemia | 3 (1.4) | 3 (1.4) | |
| Blood, other16 | 1 (0.5) | ||
| Other (n = 32) | |||
| Headache | 5 (2.3) | ||
| Eye, inflammatory | 1 (0.5) | ||
| Immune system, other17 | 4 (1.8) | 1 (0.5) | |
| Anaphylactic shock | 7 (3.2) | ||
| Kidney injury | 6 (2.7) | 1 (0.5) | |
| Kidney, other18 | 2 (0.9) | ||
| Metabolic acidosis | 3 (1.4) | 1 (0.5) | |
| Hyperthermia | 1 (0.5) | ||
1 Moderate: less than 2 % body surface.
2 Mild: lymphangitis, dysaesthesia, local ecchymosis/haematoma/discoloration, skin lesions, pruritus, local epidermolysis. Moderate: regional suffusions/haematoma/discoloration. Severe: threatening compartment syndrome.
3 Mild: creatine kinase (CK) 250–1500 U/l. Moderate: uncomplicated rhabdomyolysis, CK 1500––10,000 U/l.
4 Mild: pO2: 8.13–9.33, pCO2: 3.01–3.4; Moderate: pO2: 7.33–8.00/ pCO2: 1.87–3.00 or 6.00–7.33; Severe: pO2: <7.33/ pCO2: <1.87 or >7.33
5 Moderate: Coma Glasgow Coma Scale 8–9
6 Paralysis of the cranial nerves lll, lV and Vl
7 Mild: systolic blood pressure 150–189 mm Hg (adult) and 121–139 mm Hg (child). Severe: systolic blood pressure >190 mm Hg (adult) and >160 mm Hg (child).
8 Mild: syst. blood pressure 50–59 mm Hg (child). Moderate: systolic blood pressure 55–79 mm Hg(adult) and 40–49 mm Hg (child).
9 Mild: >100/min (adult). Moderate: 140–179/min (adult) and 160–190/min (child).
10 Moderate: 40–50/min (adult) and 60–80/min (child). Severe: <40/min (adult) and <60/min (child).
11 Partial bundle branch block.
12 Mild: thoracic pain. Moderate: collapse.
13 Mild: Quick <70% or INR >1.25. Moderate: coagulopathy without bleeding, Quick <50% or INR >1.55. Severe: venom-induced consumption coagulopathy (VICC).
14 Mild: 31,000–70,000/µl. Moderate: 11,000–30,000/µl.
15 Mild: 15,000–19,000/µl. Moderate: 20,000–39,900/µl
16 No further information
17 Anaphylactic reaction
18 Mild: urgency to urinate
Overall, 625 symptoms were reported in 219 patients. The most frequent symptoms were local effects at the bite site with mild (100, 46%) to moderate (56, 25%) swelling, pain (65, 30%) and redness (51, 23%). Gastrointestinal symptoms including nausea (31, 14%), vomiting (mild 47 (22%) and moderate 14 (6%)) and abdominal pain (25, 11%) were also frequently reported. Other systemic symptoms included cardiovascular effects, such as hypotension 20 (9%), shock 6 (3%), neurotoxicity, such as visual impairment 5 (2.3%) and haematotoxicity including coagulopathy 14 (6.5%).
Antivenom was administered in 20% of the patients (43 patients, 24 with moderate and 19 with severe symptoms) with good resolution of symptoms, although 4% of the patients required multiple doses. Available antivenoms during the study period were ViperaTab® (MicroPharm, Great Britain), an ovine antivenom manufactured with the venom of V. berus, Viperfav® (Sanofi Pasteur MSD France), an equine antivenom manufactured with the venoms of V. aspis, berus and ammodytes, and the European Viper Venom Antitoxin (Institute of Immunology Inc, Zagreb, Croatia), an equine antivenom made with V. berus and ammodytes. Of the 155 adult patients, 25 (16%) received a single dose and two (1.3%) received multiple doses of antivenom. Of the 64 children, 10 (16%) received a single dose and six (9%) multiple doses of antivenom. Nine (32%) of the patients with severe symptoms did not receive any antivenom. Most patients (80%) recovered without antivenom administration and symptomatic therapy only. Twelve (5.5%) patients developed anaphylactic reactions such as angio-oedema or urticaria (two cases and one case, respectively) to the venom (seven cases with severe symptoms such as anaphylactic shock). Two children developed mild symptoms after antivenom administration (urticaria or shivering, without need for symptomatic or specific treatment). One child suffered from hypotensive shock, but documentation did not show if symptoms occurred before or after antivenom administration. The mean duration of hospitalisation was 2 days (range 0–12), with longer hospitalisations associated with the severity of the symptoms (fig. 5).
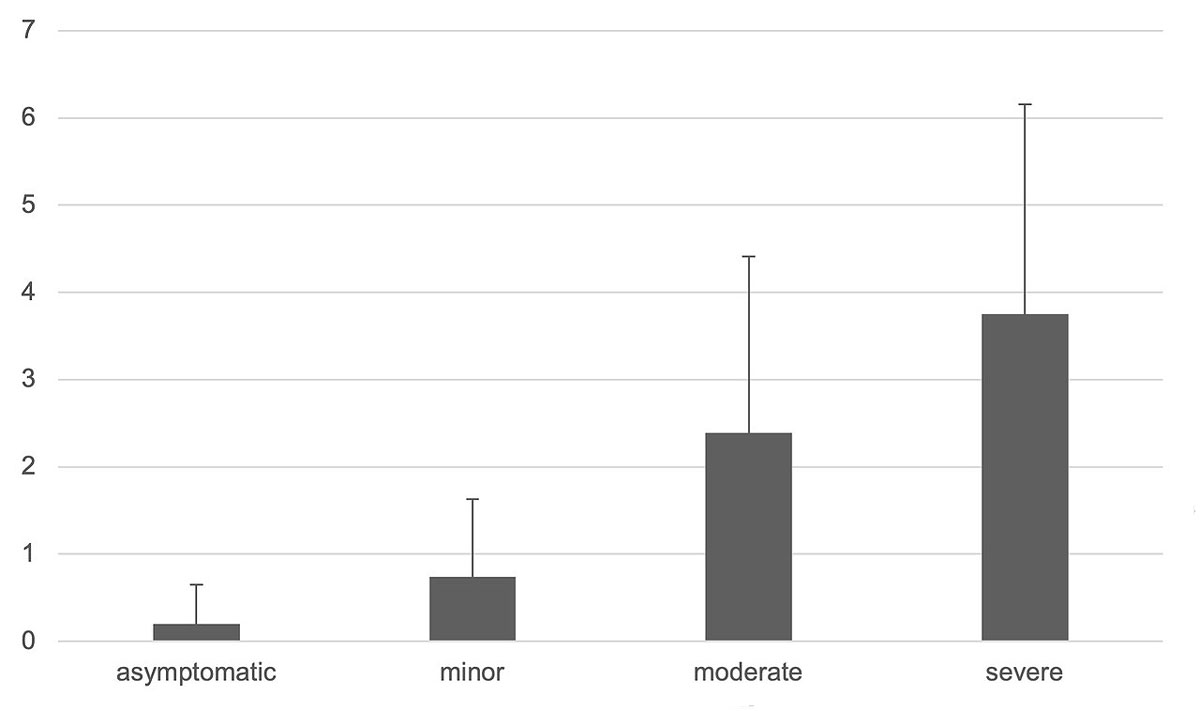
Figure 5 Mean duration of hospitalisation.
Bites by indigenous venomous snakes lead to moderate and severe symptoms in half of the patients, necessitating symptomatic treatment or antivenom. Fatal cases in adults or children were not reported within the study period. The distribution of severity of the symptoms in our patients was similar to three previous studies [7, 8, 16]. The majority of the patients experienced mild or moderate symptoms, and a small number remained asymptomatic or had a severe course. The frequent local symptoms can be explained by the venom components and the maximum concentration at the site of the bite. The composition of the venoms of V. aspis and berus is similar, although there are interregional and interspecies differences in venom composition [11, 14]. There are indications that V. aspis venom phospholipase A2 is neurotoxic, whereas V. berus venom phospholipase A2 is more haematotoxic [4, 10–13, 15], even if a level of neurotoxicity has been detected in some V. berus populations [14]. Table 4 shows some of the venom components found in both V. aspis and berus.
Table 4Venom composition of V. aspis and berus (selection, adapted from [4, 14, 15]).
| Venom component | Vipera aspis | Vipera berus |
| Kininogenase | x | x |
| Prothrombin-activating factor | x | x |
| Metalloproteinases (snake venom metalloproteinase, SVMP)1 | x | x |
| Serine proteases (snake venom serine protease, SVSP)1 | x | x |
| Hyaluronidases | x | x |
| Phospholipase A2 (PLA2) | x | x |
| Neurotoxic PLA2 (ammodytoxin A, B, C)2 | x | - |
| Neurotoxic PLA2 (vaspin)2 | x | - |
1 hematotoxic compound, 2 neurotoxic compound; the table shows a selection of compounds. In V. aspis, a total of 64 proteins were identified in transcriptomic and proteomic analyses [15]. V. berus venom has predominantly proteolytic, haemolytic and cytotoxic properties. Geographic venom variations occur within the species.
With an average of 10 bites per annum in a population of over 8 million inhabitants, indigenous snakebite has a low incidence. Yet because there is no mandatory reporting requirement, it is entirely possible that not all cases have been recorded.
The majority of patients bitten by indigenous venomous snakes were adults. The age distribution of affected patients (30% children, 70% adults) remained very comparable to two earlier studies from Switzerland [7, 18]. Results from other European countries have shown a higher proportion of children among bite victims (40–50% [16]). The reason for this difference remains unknown.
Over two thirds of the patients were male. The results in the present study related to gender distribution were similar to other epidemiologic studies [2, 7, 12, 19–21]. Males account for double of the bite victims compared with females in all age groups. The reasons for the higher proportion of men vs women as potential bite victims remain speculative. Increased outdoor activities or different risk behaviours might play a role. The median age of the patients in our study was 31 years, which was also similar to the previous study in Valais [7].
The risk of bites is increased in the summer months with the peak in July. Swiss indigenous vipers hibernate during the winter months when their habitat is usually covered with snow. Depending on the weather and the temperature, the animals become active and the risk of an encounter with humans increases [3]. Most bites happened during leisure activities, only very few were occupational exposures. These results are in contrast to data from other countries such as Kenya, where most bites happen during occupational exposure such as animal husbandry or horticulture [22].
The hand as primary site for snakebites was also observed in other studies in Switzerland [18]. In addition, our data confirmed previous data that more males than females were bitten in the hand [18, 23]. Reasons for this finding might be that males are more likely to intentionally grab the snake, try to play with it or point at it, as has been described in another study [24], although in most cases contact was unintentional (e.g., during climbing or field work). Only a smaller number of patients stepped on or too close to the snake and were consequently bitten in the foot. This is contrary to the prevalence of bites in the foot in some Nordic countries [16].
Severe symptoms occurred with adults and children in similar proportions (12% in adults and 14% in children), although a higher percentage of children (25% as opposed to 17% of the adults) received antivenom. The reason for this discrepancy remains unclear, because the indication for the administration of antivenom remains the same regardless of the age of the patient. However, the percentage of severe symptoms is comparable to other studies where the Poisoning Severity Score was used [16, 17]. Comparisons with studies using a different grading system are not feasible (for example [8, 25]). There was no difference in severity between adults and children, which was comparable to other studies [26, 27], so we suppose that factors other than age influence the outcome [27]. Yet some authors advocate a special paediatric regimen for snakebite [28]. The symptoms mostly presented as extensive regional swelling. However, severe anaphylactic reactions in 3% (seven patients, six adults and one teenager), with severe hypotension, tachycardia and angio-oedema, requiring rapid resuscitation were reported. De-novo anaphylactoid reactions have been described after snakebite possibly through auto-pharmacological effects [5].
Treatment was mostly symptomatic with hydration, elevation of the bitten limb, analgesia and tetanus vaccination in accordance with the internal and international guidelines [9]. The use of acetylsalicylic acid should be discouraged owing to its possible haematological interference, and prophylactic antibiosis or manipulation of the wound is not recommended [9]. Clinical surveillance is recommended in asymptomatic patients for at least 3–6 hours [9]. There were three children treated with fasciotomy with suspected compartment syndrome, albeit without measurement of the intracompartmental pressure, but with (repeated) administration of antivenom in all three cases. The two adults with suspected compartment syndrome did not receive antivenom before fasciotomy. In current guidelines the necessity of repeated antivenom use and measurement of intracompartmental pressure before fasciotomy is stressed [19].
Antivenom was administered to 20% of the patients. Its local availability directs the choice of antivenom. In another study from the United Kingdom, similar criteria for antivenom administration were applied, i.e., mainly refractory hypotension, protracted severe gastrointestinal symptoms, rapid extension of oedema (also of mucosae), neurological symptoms and compartment syndrome [5, 23]. Overall, 4% of the patients in the current study required multiple doses of antivenom, which was consistent with an older study [16]. In yet another study, 20% of the patients required at least a second dose [29]. The reason for this difference remains unclear, as the proportion of children was comparable.
Our study is limited by the data and data collection within a Poison Centre and the self-reporting by the physicians to the centre. As information is voluntarily reported, some clinical details may be incomplete or specific data may be subjectively reported by doctors treating the patients. The retrospective design of the study and the lack of long-term follow-up are further limitations. Prolonged injuries, adverse effects or permanent disabilities could not be tracked. Moreover, not all bites might have been reported to our centre. Especially hospital physicians, who are familiar with indigenous snakebites in various areas of Switzerland, can judge the clinical symptoms and decide on symptomatic or specific treatment options and thus might not contact the Poison Centre. However, with regard to the small annual numbers, even the expertise in local hospitals might be very limited.
Our study is the largest study conducted in Switzerland concerning national data on snakebite, distribution of severity and administration of antivenom. Our data corroborate the data of a previous study set in Switzerland in as much as half of the patients suffering a bite by an indigenous viper have an asymptomatic or mild clinical course, whereas the other half suffers from moderate or severe symptoms. There were no fatalities during the study period. In conclusion, we can say that although the situation in Switzerland can in no way compare to the massive global burden of morbidity and mortality caused by snakebite, even our indigenous vipers have the potential to cause relevant morbidity.
There was no external financial support for this study.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Factsheet WH . https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming accessed 2020 April 4.
2. Paolino G , Di Nicola MR , Pontara A , Didona D , Moliterni E , Mercuri SR , et al. Vipera snakebite in Europe: a systematic review of a neglected disease. J Eur Acad Dermatol Venereol. 2020 Oct;34(10):2247–60. https://doi.org/10.1111/jdv.16722
3. Website of the . «Koordinationsstelle für Amphibien- und Reptilienschutz in der Schweiz», www.karch.ch, accessed 2020 April 4
4. Di Nicola MR , Pontara A , Kass GE , Kramer NI , Avella I , Pampena R , et al. Vipers of Major clinical relevance in Europe: Taxonomy, venom composition, toxicology and clinical management of human bites. Toxicology. 2021 Apr;453:152724. https://doi.org/10.1016/j.tox.2021.152724
5. Meier J , Berney C . Aspisviper (Vipera aspis) und Kreuzotter (Vipera berus): Die medizinisch bedeutsamen Giftschlangen der Schweiz. 1. Teil: Biologie, Verbreitung und Giftzusammensetzung. Swiss Med Forum. 2003;03(32):746-753.
6. Plate A , Kupferschmidt H , Schneemann M. Giftschlangenbisse in der Schweiz. [Bites of venomous snakes in Switzerland]. Praxis (Bern 1994). 2016;105(12):679-85; quiz 684-5. doi: https://doi.org/10.1024/1661-8157/a002388.
7. Petite J . Viper bites: treat or ignore? Review of a series of 99 patients bitten by Vipera aspis in an alpine Swiss area. Swiss Med Wkly. 2005 Oct;135(41-42):618–25.
8. Audebert F , Sorkine M , Bon C . Envenoming by viper bites in France: clinical gradation and biological quantification by ELISA. Toxicon. 1992 May-Jun;30(5-6):599–609. https://doi.org/10.1016/0041-0101(92)90854-X
9. Meier J , Rauber-Lüthy C , Kupferschmidt H . Aspisviper (Vipera aspis) und Kreuzotter (Vipera berus): Die medizinisch bedeutsamen Giftschlangen der Schweiz - 2.Teil: Vorbeugung, Erste Hilfe und Behandlung von Bissunfällen. Swiss Med Forum. 2003;03(34):780-785. doi: https://doi.org/10.4414/smf.2003.04950
10. Ferquel E , de Haro L , Jan V , Guillemin I , Jourdain S , Teynié A , et al. Reappraisal of Vipera aspis venom neurotoxicity. PLoS One. 2007 Nov;2(11):e1194. https://doi.org/10.1371/journal.pone.0001194
11. Zanetti G , Duregotti E , Locatelli CA , Giampreti A , Lonati D , Rossetto O , et al. Variability in venom composition of European viper subspecies limits the cross-effectiveness of antivenoms. Sci Rep. 2018 Jun;8(1):9818. https://doi.org/10.1038/s41598-018-28135-0
12. Jollivet V , Hamel JF , de Haro L , Labadie M , Sapori JM , Cordier L , et al. European viper envenomation recorded by French poison control centers: A clinical assessment and management study. Toxicon. 2015 Dec;108:97–103. https://doi.org/10.1016/j.toxicon.2015.09.039
13. Lonati D , Giampreti A , Rossetto O , Petrolini VM , Vecchio S , Buscaglia E , et al. Neurotoxicity of European viperids in Italy: Pavia Poison Control Centre case series 2001-2011. Clin Toxicol (Phila). 2014 Apr;52(4):269–76. https://doi.org/10.3109/15563650.2014.904046
14. Malina T , Krecsák L , Korsós Z , Takács Z . Snakebites in Hungary—epidemiological and clinical aspects over the past 36 years. Toxicon. 2008;51(6):943-51. RE:view. https://doi.org/10.1016/j.toxicon.2007.12.001
15. Giribaldi J , Kazandjian T , Amorim FG , Whiteley G , Wagstaff SC , Cazals G , et al. Venomics of the asp viper Vipera aspis aspis from France. J Proteomics. 2020 Apr;218:103707. https://doi.org/10.1016/j.jprot.2020.103707
16. Karlson-Stiber C , Salmonson H , Persson H . A nationwide study of Vipera berus bites during one year-epidemiology and morbidity of 231 cases. Clin Toxicol (Phila). 2006;44(1):25–30. https://doi.org/10.1080/15563650500394597
17. Persson HE , Sjöberg GK , Haines JA , Pronczuk de Garbino J . Poisoning severity score. Grading of acute poisoning. J Toxicol Clin Toxicol. 1998;36(3):205–13. https://doi.org/10.3109/15563659809028940
18. Stahel E , Wellauer R , Freyvogel TA . Vergiftungen durch einheimische Vipern (Vipera berus und Vipera aspis). Eine retrospektive Studie an 113 Patienten [Poisoning by domestic vipers (Vipera berus and Vipera aspis). A retrospective study of 113 patients] [German.]. Schweiz Med Wochenschr. 1985 Jun;115(26):890–6.
19. Ruha AM , Kleinschmidt KC , Greene S , Spyres MB , Brent J , Wax P , et al.; ToxIC Snakebite Study Group . Snakebite Study Group. The epidemiology, clinical course, and management of snakebites in the North American Snakebite Registry. J Med Toxicol. 2017 Dec;13(4):309–20. https://doi.org/10.1007/s13181-017-0633-5
20. Boels D , Hamel JF , Bretaudeau Deguigne M , Harry P . European viper envenomings: assessment of Viperfav™ and other symptomatic treatments. Clin Toxicol (Phila). 2012 Mar;50(3):189–96. https://doi.org/10.3109/15563650.2012.660695
21. Frangides CY , Koulouras V , Kouni SN , Tzortzatos GV , Nikolaou A , Pneumaticos J , et al. Snake venom poisoning in Greece. Experiences with 147 cases. Eur J Intern Med. 2006 Jan;17(1):24–7. https://doi.org/10.1016/j.ejim.2005.10.001
22. Ochola FO , Okumu MO , Muchemi GM , Mbaria JM , Gikunju JK , Onono JO , et al. Epidemiology of snake bites in selected areas of Kenya. Pan Afr Med J. 2018 Apr;29:217. https://doi.org/10.11604/pamj.2018.29.217.15366
23. Lamb T , Stewart D , Warrell DA , Lalloo DG , Jagpal P , Jones D , et al. Moderate-to-severe Vipera berus envenoming requiring ViperaTAb antivenom therapy in the UK. Clin Toxicol (Phila). 2021 Nov;59(11):992–1001. https://doi.org/10.1080/15563650.2021.1891245
24. Jaramillo JD , Hakes NA , Tennakoon L , Spain D , Forrester JD . The “T’s” of snakebite injury in the USA: fact or fiction? Trauma Surg Acute Care Open. 2019 Oct;4(1):e000374. https://doi.org/10.1136/tsaco-2019-000374
25. Valenta J , Stach Z , Stříteský M , Michálek P . Common viper bites in the Czech Republic - epidemiological and clinical aspects during 15 year period (1999-2013). Prague Med Rep. 2014;115(3-4):120–7. https://doi.org/10.14712/23362936.2014.42
26. Chippaux JP , Saz-Parkinson Z , Amate Blanco JM . Epidemiology of snakebite in Europe: comparison of data from the literature and case reporting. Toxicon. 2013 Dec;76:206–13. https://doi.org/10.1016/j.toxicon.2013.10.004
27. Levine M , Ruha AM , Wolk B , Caravati M , Brent J , Campleman S , et al.; ToxIC North American Snakebite Study Group . North American Snakebite Study Group. When it comes to snakebites, kids are little adults: a comparison of adults and children with Rattlesnake bites. J Med Toxicol. 2020 Oct;16(4):444–51. https://doi.org/10.1007/s13181-020-00776-6
28. Marano M , Pisani M , Zampini G , Pontrelli G , Roversi M . Acute exposure to European viper bite in children: advocating for a pediatric approach. Toxins (Basel). 2021 May;13(5):330. https://doi.org/10.3390/toxins13050330
29. Personne M , Hulten P . The need of a second antivenom dose after snake bites by Vipera berus. (2017) 37th International Congress of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) 16–19 May 2017, Basel, Switzerland, Clin Toxicol (Phila). 55:5, 488, doi: https://doi.org/10.1080/15563650.2017.1309792