Functional verification of the JmLFY gene associated with the flowering of Juglans mandshurica Maxim.
- Published
- Accepted
- Received
- Academic Editor
- Sajid Fiaz
- Subject Areas
- Agricultural Science, Molecular Biology, Plant Science
- Keywords
- Juglans mandshurica Maxim., JmLFY gene, Functional verification
- Copyright
- © 2023 Cai et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Functional verification of the JmLFY gene associated with the flowering of Juglans mandshurica Maxim. PeerJ 11:e14938 https://doi.org/10.7717/peerj.14938
Abstract
In this study, a pBI121-JmLFY plant expression vector was constructed on the basis of obtaining the full-length sequence of the JmLFY gene from Juglans mandshurica, which was then used for genetic transformation via Agrobacterium inflorescence infection using wild-type Arabidopsis thaliana and lfy mutants as transgenic receptors. Seeds of positive A. thaliana plants with high expression of JmLFY were collected and sowed till the homozygous T3 regeneration plants were obtained. Then the expression of flowering-related genes (AtAP1, AtSOC1, AtFT and AtPI) in T3 generation plants were analyzed and the results showed that JmLFY gene overexpression promoted the expression of flowering-related genes and resulted in earlier flowering in A. thaliana. The A. thaliana plants of JmLFY-transformed and JmLFY-transformed lfy mutants appeared shorter leaves, longer fruit pods, and fewer cauline leaves than those of wild-type and the lfy mutants plants, respectively. In addition, some secondary branches in the transgenic plants converted into inflorescences, which indicated that the overexpression of JmLFY promoted the transition from vegetative growth to reproductive growth, and compensate the phenotypic defects of lfy mutant partially. The results provides a scientific reference for formulating reasonable genetic improvement strategies such as shortening childhood, improving yield and quality, and breeding desirable varieties, which have important guiding significance in production.
Introduction
Flowering is an important process in the life cycle of higher plants. The developmental processes of flowers are divided into flowering decisions, flower initiation, and flower organ development (Zhang & Liu, 2003). During plant flower induction, the external environment and genetic factors together form a complex regulatory network (Zeng et al., 2018; Hassankhah et al., 2018). The model plant Arabidopsis thaliana controls flowering through the interaction of temperature, vernalization, photoperiod, gibberellin, and autonomous pathways (Martina, Nadine & Christian, 2015; Jian et al., 2019; Jin et al., 2019).
In the flowering regulatory network of plants, the LEAFY (LFY) gene is a key transcription factor in the flowering signaling pathway, which is expressed in the early stage of flower primordia initiation. The LFY gene determines the floral meristem properties of the flower primordia by activating the expression of floral meristem characteristic genes and floral organ characteristic genes, such as AP1 and CAL. Therefore, the LFY gene is an important floral meristem characteristic gene (Alvarez-Buylla, Garcia-Ponce & Garay-Arroyo, 2006; He et al., 2018). LFY and other genes related to flower formation together form a flower development regulatory network and jointly control plant flower formation. The AtAP1 gene is involved in the formation of flower meristem, which is a characteristic gene of flower organ and plays an important role in the process of flower formation. AP1 and LFY genes are positively regulated by each other. SOC1 gene is the upstream gene of LFY gene, which can integrate multiple flowering pathways; the FT gene is also the upstream gene of LFY gene and the target gene of CO protein; the PI gene is the downstream gene of the LFY gene, and the LFY gene promotes the expression of PI gene (Zhao et al., 2020).
The LFY gene plays an important role not only for controlling the transition from inflorescence meristem to floral meristem but also for regulating flowering time in A. thaliana (Weigel et al., 1993; Weigel & Nilsson, 1995; He, Wang & Zhang, 2011; He et al., 2017). When the LFY gene was transferred into wild-type A. thaliana, it was found that all lateral shoots were transformed into flowers (Blazquez et al., 1997). In a functional study of LFY genes in plants such as Populus simonii, Nicotiana tabacum, and Oryza sativa, it was found that transfection and overexpression of LFY gene could lead to earlier flowering (He et al., 2000; Ahearn et al., 2001; Peña et al., 2001). The activity of the LFY gene is conserved even in distantly related species (Shao et al., 1999). Therefore, LFY is not only a key regulatory gene in the plant flowering pathway but also a key regulator of downstream floral meristem and floral organ-determining genes (Feng, Li & Wang, 2016). Although the LFY gene has been isolated and cloned in many plants, functional studies have shown that it plays an important role in the reproductive and growth processes of plants. However, no studies on the LFY homologous gene in J. mandshurica have been conducted.
Juglans mandshurica is a dichogamous and monoecious plant with two main mating types, protandrous and protogynous. The asynchronous development and the imbalanced ratios of the female and male flowers affect the efficiency of pollination and fruit setting (Zhang et al., 2019). For the walnut species, the fruit production is also limited by late-spring frost in many countries (Hassankhah et al., 2017), thus the late-leafing and early-harvesting varieties have been studied in Juglans regia by targeted hybridization, and a significant variation between seedlings in terms of leafing date (45 days) were observed, which indicated that it is possible to get late leafing and early-harvesting genotypes with desirable nut traits (Hassankhah et al., 2017; Fallah et al., 2022). Currently, there are few studies focus on the development of male and female flower buds and the asynchrony of flowering period. In J. regia, the flowering pattern was altered by spraying GA3 (the best concentration is 100 mg/L), which increased the total number of flowers, the male flowers, and male: female flower ratio significantly (Hassankhah et al., 2018). However, the molecular mechanism of flower formation in the process of male and female differentiation has not been fully analyzed.
Therefore, based on the successful cloning of the JmLFY gene in the early stage of the experiment, a plant expression vector of the JmLFY gene of J. mandshurica was successfully constructed and transformed with Agrobacterium to obtain transgenic A. thaliana plants, and the T0–T3 generations of the transgenic plants were verified and analyzed for the function of the JmLFY gene. This study lays a foundation for the exploration of the molecular mechanism of the flowering of J. mandshurica, and provides a scientific basis for the shortening of the juvenile period, early flowering, yield and quality improvement of J. mandshurica, selection of improved varieties, and rational formulation of genetic improvement strategies.
Materials and Methods
Plant material
The test materials were obtained from the JmLFY gene strain stored in the Forest Genetics and Breeding Laboratory of Shenyang Agricultural University and stored at −80 °C; wild-type A. thaliana seeds (Columbia type) and lfy mutant seeds were purchased from TAIR website (https://www.arabidopsis.org/index.jsp).
Experimental reagents
BM Seamless Cloning Kit (Biomed, Beijing, China), DH5 α and Agrobacterium GV3101(Biomed, Beijing, China), small plasmid extraction kit (TIANGEN, Sichuan, China), Gel recovery, DL2000 Marker, High fidelity enzyme and LA Taq (TaKaRa, Beijing, China), X-gal and IPTG (Real Times, Shenzhen, China), Bis, Tris Hcl, SDS, TEMED, ammonium persulfate, ammonium persulfate, kana, sucrose, and agar powder (Sinopharm, Beijing, China), and LB medium (ShengGong, Liaoning, China).
Acquisition of the JmLFY gene of J. mandshurica
The plasmid was extracted from the JmLFY cDNA bacterial solution of J. mandshurica stored at −80 °C, and the full-length PCR gene was amplified using high-fidelity enzymes and specific primers, the primer sequences are shown in Table 1. The PCR products were recovered by cutting the gel using a gel recovery kit and then were sent to Goldwisdom for sequencing.
| Primer | Sequence (5′-3′) | Use |
|---|---|---|
| LFY-F2 | CACGGGGGACTCTAGAATGGATCCCGACCCCTTTACTG | JmLFY vector construction |
| LFY-R2 | AGGGACTGACCACCCGGGTAGAGGGGCATGTGATCACCC | JmLFY vector construction |
| LP | GAGGAGGAAGTGGTTACTGGG | lfy mutant validation |
| RP | AAATGCCTACCAAAATTATAACCG′ | lfy mutant validation |
| LBb1.3 | ATTTTGCCGATTTCGGAAC | lfy mutant validation |
| JmLFY-F2 | AGCACCCTTTCATTGTAACGGA | JmLFY expression analysis |
| JmLFY-R2 | GTGCTGCTATGGCGACCAAAG | JmLFY expression analysis |
| Actin-F | ATGCCCAGAAGTCTTGTTCC | JmLFY expression analysis |
| Actin-R | TGCTCATACGGTCAGCGATA | JmLFY expression analysis |
| AtAP1-F | GCTCTTAAGGCACATCCGCAC | JmLFY functional verification |
| AtAP1-R | GCAGAGGGGGAGGCATATTG | JmLFY functional verification |
| AtFT-F | ATGCCCAGAAGTCTTGTTCC | JmLFY functional verification |
| AtFT-R | TGCTCATACGGTCAGCGATA | JmLFY functional verification |
| AtSOC1-F | AGCAGAGTTGTTGGAGACGTT | JmLFY functional verification |
| AtSOC1-R | AGGTGAGGGTTGCTAGGACT | JmLFY functional verification |
| AtPI-F | TACAACTGGAGCTCAGGCAT | JmLFY functional verification |
| AtPI-R | ATTCCTCTTGCGTTGCTTG | JmLFY functional verification |
Construction of JmLFY gene expression vector
The plasmid pBI121 was double-digested with XbaI and SmaI, and the gel-recovered product was ligated into the pBI121 vector. The recombinant plasmid pBI121-JmLFY was transformed into DH5α cells and detected by PCR. The positive bacterial liquid that was successfully verified by sequencing was subjected to plasmid extraction, transformed into Agrobacterium GV3101 competent cells, and sent to Goldwisdom for sequencing after PCR. The successfully detected bacterial solution was used to prepare an infection solution.
Validation of lfy mutants
Resistance validation of lfy mutants of A. thaliana was conducted by kanamycin (KAN) screening, for which seeds of lfy mutants were cultured on 1/2 MS + 15 g/L sucrose + 10 g/L agar + 50 μg/mL KAN. The seeds were cultured at 4 °C for 3 d and then transferred into climatic cabinate, which maintained relative humidity of 60%, temperature of 20–22 °C, light cycle of 24 h and light intensity is 80–200 μmol/m2/s.
Homozygosity validation of lfy mutants of A. thaliana was conducted by three-primer method, primers were designed using T-DNA Primer Design (http://signal.salk.edu/tdnaprimers.2.html), the sequences of the three primers are listed in Table 1. DNA from leaves of wild type and lfy mutants of A. thaliana were extracted using TIANGEN Plant Genomic DNA Kit, which were then used for PCR. The PCR products were then detected by agarose gel electrophoresis.
Agrobacterium-mediated genetic transformation of pBI121-JmLFY to A. thaliana
Genetic transformation of pBI121-JmLFY was conducted by inflorescence infection. Seeds of wild-type and lfy mutants of A. thaliana were sterilized and cultured in 1/2 MS medium, and the seedlings were selected and transplanted after 7 to 10 days of germination into the culture soil with robust and consistent growth (Fig. 1A). After bolting and flowering (Fig. 1B), the small pots were inverted, and all the inflorescences were placed upside down in the prepared infection solution, which is described in “Construction of a JmLFY gene expression vector” (Fig. 1C). The transformation was performed every three days and the process was repeated four times, and the mature seeds (Fig. 1D) were collected for obtained regenerated plants.
Figure 1: Genetic transformation of Arabidopsis thaliana.
(A) Arabidopsis sowing. (B) Arabidopsis flowering. (C) inflorescence infection process. (D) Arabidopsis seed collection.Screening of positive plants
The seeds received above were screened on 1/2 MS + KAN plates, and plants with KAN resistance were preliminarily screened. The screened plants were transferred onto the soil, and after 15 days of bolting, the DNA and RNA of the leaves were extracted using DNA and RNA extraction kits (TIANGEN, Sichuan, China), and reverse transcribed into cDNA using a cDNA synthesis kit (ShengGong, Liaoning, China), using cDNA as the template for PCR detection, and successfully detected by qRT-PCR using 2 × SG Fast qPCR master mix (Lablead).
Overexpression of JmLFY gene and regulation of other genes related to flowering
The plants with high expression of JmLFY were harvested and cultured to the T3 generation using the same method described above. The plants with the highest expression of JmLFY were selected from the T3 generation plants, and the actin gene of A. thaliana was used as the internal reference gene to determine the expression of the flowering-related genes AtAP1, AtFT, AtSOC1, and AtPI. The qRT-PCR primers used are listed in Table 1.
Morphological observation and statistical analysis
The selected LFY transgenic A. thaliana plants were named L1–L10, and the LFY transgenic lfy mutant plants were named C1–C10. The L-line and C-line plants with high expression of JmLFY were selected and cultured simultaneously with the wild-type and lfy mutants, and the bolting, flowering, and fruit pod formation times of the four lines were observed and recorded. The number of rosette leaves, plant height, and leaf shape and size were recorded and statistical analyses were performed using SPSS and Excel.
Results
Acquisition of the JmLFY gene related to the flowering of J. mandshurica
The plasmid was extracted from the JmLFY cDNA bacterial solution of J. mandshurica stored at −80 °C in our laboratory. Using the JmLFY gene plasmid as the template, the upstream and downstream primers of the JmLFY gene were designed according to the obtained CDS sequence of the JmLFY gene, and the full-length gene was amplified by PCR. The PCR amplification products were sent to Goldwisdom for sequencing, and the sequencing results showed that the JmLFY target gene was successfully obtained from the cDNA-glycerol bacteria. The JmLFY sequence is shown in Fig. 2.
Figure 2: Amplification of the JmLFY gene related to flowering in Juglans mandshurica.
Construction of the pBI121-JmLFY plant expression vector
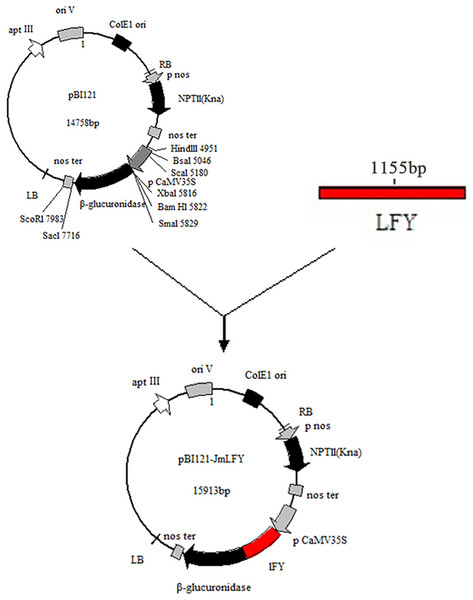
The PCR-amplified product of the JmLFY gene was cut into a gel and ligated with the pBI121 vector. Sequencing and comparison showed that the homology between the two cDNA sequences reached 99.91%. The resulting plant expression vector was named pBI121-JmLFY and mapped (Fig. 3).
Figure 3: Construction of plant expression vector pBI121-JmLFY.
lfy mutant validation
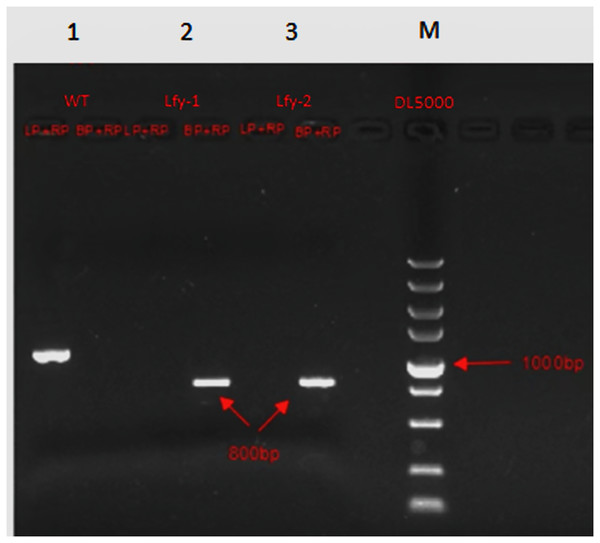
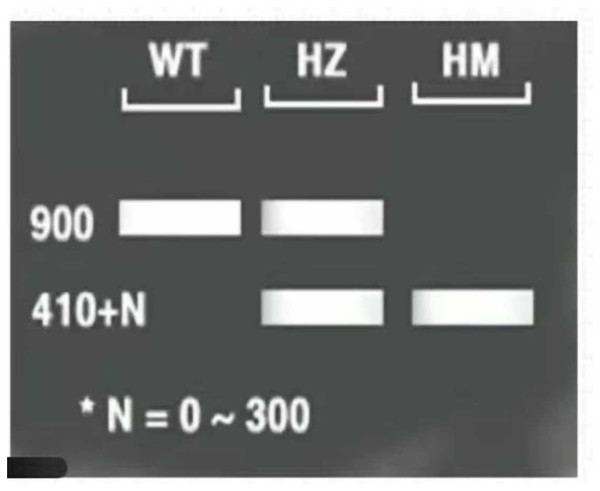
The three-primer method was used to verify the homozygosity of A. thaliana lfy mutants, and a 1/2 MS KAN plate was used to screen the A. thaliana lfy mutants for resistance. As shown in Fig. 4, the length of the wild-type band was 900 bp and that of the lfy mutant A. thaliana was approximately 800 bp. It can be concluded that the lfy mutant is homozygous for Arabidopsis, which is in line with the expected results (Fig. 5). After screening on a 1/2 MS KAN plate, it was found that the A. thaliana lfy mutant had yellow leaves, could not grow roots and true leaves, and could not survive for a long time (Fig. 6), indicating that it was no KAN resistant and could be used for subsequent transformation validation experiments.
Figure 4: Homozygous verification of Arabidopsis.
Figure 5: Expected results for Arabidopsis 1–3: PCR products; M: DL5000 marker; WT: wild Arabidosis; HZ: heterozygote; and HM homozygous verification of Arabidopsis.
Figure 6: Verification of kana resistance of lfy mutant.
Phenotypic observation showed that the inflorescence of the lfy mutant was transformed into secondary branches, with absent petals and incomplete flower development (Fig. 7), while the lfy mutant plants were highly sterile (Zhang et al., 2008).
Figure 7: (A–B) lfy mutant phenotype with secondary branch.
Screening of positive plants
Wild-type and lfy mutant A. thaliana transformed into the pBI121-JmLFY vector were screened for KAN plate resistance. The positive plants grew true leaves and took root on the KAN plate, while false positives did not (Fig. 8). At approximately 20 days, they were transferred to the prepared matrix soil. Five seedlings with the best growth were selected, and the selected JmLFY transgenic wild-type A. thaliana were named L-line plants while the selected lfy mutants A. thaliana were named C-line plants.
Figure 8: Positive and false positive plants screened from the T0 generation.
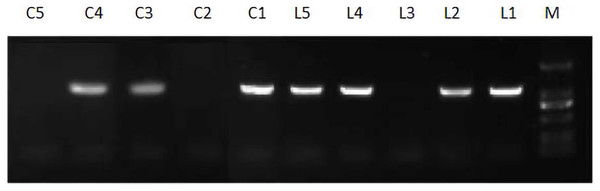
After flowering, the leaves were collected from the preliminarily screened lines, and the extracted RNA was reverse-transcribed into cDNA for positive PCR detection. The test results are shown in Fig. 9. L1, L2, L4, L5 and C1, C3, and C4 were found to be positive. The transformation rates of these two strains were not the same, which may be related to the quality of the recombinant plant expression vector and the operation method.
Figure 9: Expression of JmLFY transgenic plants.
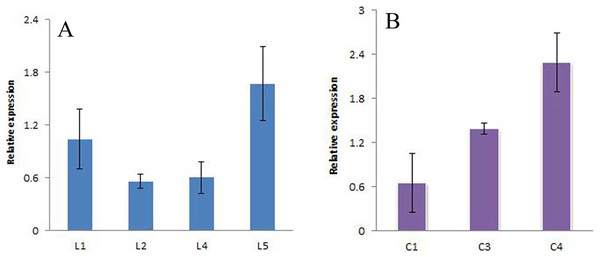
The positive seedlings screened above were subjected to qRT-PCR analysis, and A. thaliana Actin was used as the internal reference gene to analyze the expression of the JmLFY gene in different lines (Fig. 10 and Supplemental File (raw data)). The C4 expression levels were found to be higher. The four harvested strains were continuously cultivated to the T3 generation, and C23, C28, and C7, which had the highest JmLFY gene expression, were screened for further verification.
Figure 10: Expression of JmLFY gene in different parts.
(A) JmLFY transgenic plant. (B) JmLFY transgenic plants with lfy mutant.Regulation of JmLFY gene on other flowering-related genes
To further explore the regulation of other flowering-related genes in A. thaliana by the JmLFY gene of J. mandshurica, Primer-BLAST was used to design quantitative primers, and fluorescence quantitative analysis was used to analyze the expression of each flower-related gene in the JmLFY transgene, JmLFY-transfected lfy mutant, and wild type. Differences in foreign gene expression were found in different transgenic lines (Fig. 11 and Supplemental File (raw data)).
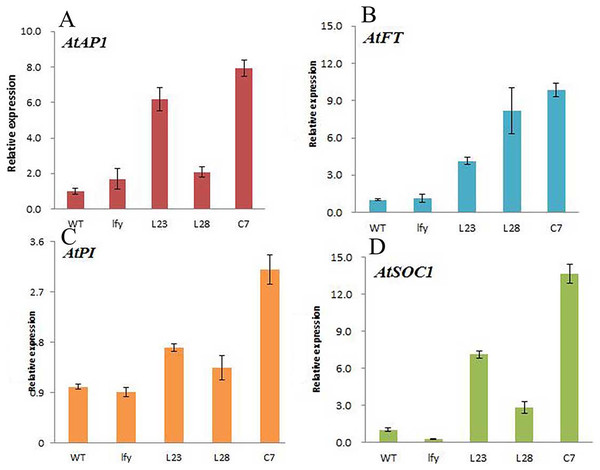
Figure 11: Expression of different flowering genes in JmLFY transgenic plants.
(A) AtAP1 gene. (B) AtSOC1 gene. (C) AtPI gene. (D) AtFT gene.Compared to the wild type, the expression levels of all the genes related to flower formation in Arabidopsis plants transformed with the JmLFY gene changed, although the changes were different. The expression level of the AtAP1 gene was significantly increased in L23 and C7 plants, but did not change significantly in the L28 line (Fig. 11A). The expression level of AtSOC1 was significantly increased in L23 and C7 plants. The expression level in the L28 line was also increased, and was much higher than in wild-type A. thaliana (Fig. 11B). The expression of AtFT gene was significantly increased in the L23, L28, and C7 (Fig. 11D); the expression of AtPI gene in L23 and L28 lines did not change significantly, although the expression level was significantly increased in the C7 line (Fig. 11C).
The flower bud formation time, flowering time, number of rosette leaves, plant height, and number of secondary branches in the T3 generation lines are shown in Table 2 and uploaded as a Supplemental File (raw data). The phenotypes of the four lines were then compared (Fig. 12). The results showed that the flowering time of the transgenic JmLFY plants was significantly earlier than that of the wild type; approximately eight days earlier, the rosette leaves of the L23 and L28 plants were approximately four times lower than those of the wild type, and the plant height, number of secondary branches, and number of cauline leaves were higher than those of the wild type. Compared to the lfy mutant, there was no obvious change, and the flowering time of the transfected lfy mutant was still later than that of the wild-type A. thaliana; however, it was also significantly earlier than that of the lfy mutant by approximately 7 days. The number of rosette leaves in the C7 plant was higher than that of the lfy mutant. Approximately five fewer leaves, four fewer secondary branches, and five fewer cauline leaves (Table 2); transgenic plants have shorter leaves and longer pods than the wild type (Figs. 12H and 12I), and all shoots were transformed into single flowers several times (Fig. 12F), indicating that JmLFY, as a characteristic gene of floral meristem, can control the transition from vegetative growth to reproductive growth. lfy mutants are sterile plants, and the flowers remained intact after the transfer of the JmLFY gene (Fig. 12G). Most of the flowers regained their normal morphology, produced more seeds than the lfy mutant, and produced fewer rosette leaves, secondary shoots, and cauline leaves than the lfy mutant (Fig. 12J). Some secondary branches were also transformed into single flowers, and JmLFY also transformed secondary branches into inflorescences. In addition, JmLFY regulates the expression of other flowering-related genes in A. thaliana and promotes the expression of these genes, indicating that the function of JmLFY may also be related to the regulation of these genes. These results illustrate the important role of JmLFY in flower formation.
| Strain | Number of rosette leaves | Flower bud formation time | Flowering time | Plant height | Number of secondary branches | Number of stem leaves |
|---|---|---|---|---|---|---|
| WT | 13.8 ± 0.84c | 31.4 ± 1.14c | 37.4 ± 0.55c | 25.92 ± 1.12b | 4.2 ± 0.84c | 5.4 ± 1.14c |
| lfy | 23.4 ± 1.14a | 41.6 ± 1.14a | 49.8 ± 0.84a | 29.5 ± 0.46a | 12.4 ± 1.14a | 16.8 ± 0.84a |
| L23 | 10.2 ± 0.44d | 22.8 ± 0.84d | 28.8 ± 0.84d | 25.98 ± 1.33b | 4 ± 0.71c | 4.4 ± 1.14cd |
| L28 | 9.6 ± 0.89d | 23.2 ± 0.44d | 29.2 ± 0.84d | 26.46 ± 0.48b | 4.42 ± 0.84c | 4 ± 0.71d |
| C7 | 18.8 ± 0.84b | 37.4 ± 0.55b | 41.2 ± 0.84b | 25.7 ± 0.64b | 7.6 ± 0.89b | 11.4 ± 0.55b |
Note:
The values are the means ± standard errors. Different letters in the same column indicate significant differences at P = 0.05 level.
Figure 12: Phenotypic study of overexpression of JmLFY gene in Arabidopsis.
(A) lfy mutant; (B) WT; (C) L23; (D) left: WT, right: L23; (E) transgenic lines flowering ahead of time; (F) transformation from secondary branch to single flower L28 (G) C7 flower; (H) up: WT down: L28; (I) WT (left) L28 (right); (J) C7 stem leaf.Discussion
The transition from vegetative to reproductive growth is an important process in the plant life cycle, and many physiological and metabolic changes and gene regulation mechanisms are involved in this process (Saquib & Li, 2020; Dong et al., 2021). These gene regulatory networks do not exist independently but interact and regulate each other to jointly regulate the flowering process of plants (Lu et al., 2020; Kalve et al., 2020). The LFY homologous gene regulates the formation of floral meristems and organs and is critical for flowering (Bosl et al., 2004). As a transcription factor, LFY regulates the transition of plants from vegetative growth to reproductive growth and is mainly expressed in the apical meristem. Under the control of the 35S promoter, LFY overexpression promotes early flowering and transforms secondary shoots into single flowers (Zhang et al., 2008). Lfy mutants are defective in flowering and inflorescence formation (Mori et al., 2017; Ma et al., 2020). When GhLFY is overexpressed in A. thaliana lfy-5 mutants, lateral shoot secondary buds are transformed into individual flowers and the wild-type flower phenotype is restored (Tang et al., 2016). In this study, the secondary shoots of the transgenic A. thaliana were transformed into single flowers, the mutant plants of the transgenic JmLFY gene exhibited normal petals, the phenotype of the mutant plants was restored to normal, and the seeds produced are more and better quality than the lfy mutant. These results are similar to those of mutant lfy overexpressing the JcLFY gene of Jatropha curcas (Tang et al., 2016) and the EgLFY gene of Eucalyptus grandis (Dornelas & Rodriguez, 2006), indicating that the JmLFY gene can complement the late-flowering phenotype of lfy mutants and promote the transition from vegetative growth to reproductive growth.
Plant flower bud differentiation is a complex physiological process regulated by multiple genes (Zhang et al., 2019). In J. regia, expression of FT gene activated downstream floral meristem identity genes including SOC1, and LFY which consequently led to release bud dormancy as well as flower anthesis and induction (Hassankhah et al., 2020). As a master regulator of flowering and floral gene networks, LFY activates downstream floral meristem recognition genes (AP1 and CAL) (Zou et al., 2014). AP1 is a floral meristem signature gene and a direct target of LFY (Ma et al., 2020). AP1 mRNA gradually accumulates after LFY expression (Haughn, 1991). In addition, LFY also promotes the expression of floral organ-specific recognition genes, such as PI, AG, and AP3, as well as the E functional genes SEP1, SEP2, and SEP3. In this study, compared with the wild type, the expression of AtAP1 gene was significantly increased in L23 and C7 plants, but did not significantly change in the L28 line; the expression of AtSOC1 gene was significantly increased in L23 and C7 plants, and the expression level of the L28 line was also higher than that of wild-type A. thaliana. The expression level of the AtFT gene in L23 and L28 was significantly increased; and the expression level of AtPI gene in L23 and L28 lines showed no significant change; however, the expression level was significantly increased in the C7 line. This indicated that the three genes AtAP1, AtSOC1, and AtPI may contribute to the normal flower morphology of C7.
Transferring JcLFY into Jatropha curcas caused transgenic plants to bloom seven months earlier (Tang et al., 2016). Peña et al. (2001) transformed the AtLFY and AtAP1 genes into Citrus sinense and Poncirus trifoliata, and found that transformed plants flowered 3–5 years earlier than untransformed plants (Pineiro, 1998; He, Wang & Zhang, 2011; Ma et al., 2019). In the present study, the transgenic A. thaliana L23 and L28 lines flowered six days earlier than the wild-type plants, and the C7 line flowered eight days earlier than the corresponding mutant lfy. These results suggest that JmLFY promotes early flowering in A. thaliana, shortens the vegetative phase, and complements the delayed flowering of the mutant lfy. In addition, the L23 and L28 lines had fewer rosette and cauline leaves than the wild type. And also, the number of clump leaves, cauline leaves, and branches of the transgenic line C7 were higher than those of the wild-type plants but lower than those of the lfy mutant. The results obtained in this study are in agreement with those obtained in A. thaliana (Weigel & Nilsson, 1995), New Zealand radiata pine (Vazquez et al., 2007), rapeseed (Roy, Saxena & Bhalla, 2009) and Jatropha curcas. The phenotypes of plants overexpressing the LFY gene in Tang et al. (2016) were similar. These results confirm that JmLFY can inhibit the vegetative growth of plants, partially complement the phenotypic defects of lfy mutants, and promote the earlier flowering of plants. And in the future research, the Southern Blot method should be considered to better confirm the transgenic plants.
Conclusions
In this study, the plant expression vector pBI121-JmLFY was successfully constructed using JmLFY cDNA bacterial solution. The three-primer method and KAN resistance screening confirmed that the lfy mutant was homozygous and did not exhibit KAN resistance. Phenotypic observations showed that the inflorescence of lfy mutant Arabidopsis was transformed into secondary branches, with absent petals and incomplete flower development. The lfy mutant plants were highly sterile and produced few seeds. pBI121-JmLFY was transformed into wild-type and lfy mutants to obtain regenerated plants, and the JmLFY-transgenic Arabidopsis and JmLFY-transgenic Arabidopsis were screened for positive seedlings using KAN plates. Expression of the JmLFY gene was screened and plants with the highest levels of expression were those from the L23, L28, and C7 lines. Validation of the T3 generation plants showed that JmLFY also regulated the expression of other flowering-related genes in A. thaliana and promoted the expression of these genes. The JmLFY gene can partially compensate for the phenotypic defects of lfy mutants, and promote transition from vegetative to reproductive growth and early flowering.