Abstract
The extract of green algae (Enteromorpha species) was prepared by the cold extraction technique. The prepared algal extract exhibits a high antioxidant potential due to the presence of sulfated polysaccharides (SPs). The extract of Enteromorpha species was analyzed to identify the presence of significant biochemical composition. The extract of Enteromorpha species was evaluated to assess the DPPH-free radical scavenging activity, total antioxidant activity by phosphomolybdenum assay, in vitro anti-bacterial by agar diffusion method, and cell viability by MTT assay. It was found that the extract of Enteromorpha species contains the various chemical composition such as carbohydrates (0.13 g/ml), xylose (0.0819 g/ml), sulfate (0.0153 g/ml), and proteins (0.0363 g/ml). Phytochemicals such as flavonoids and phenolic compounds were found in the extract. The antioxidant potential of the crude extract was investigated by the total antioxidant assay (400 µl/ml) and DPPH-free radical scavenging assay (5 µl/ml). The prepared green algal extract produced the highest inhibitory zone up to 18 mm, 13 mm, and 18 mm at 200 µl/ml concentrations against Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli, respectively. The above results revealed that the extract of Enteromorpha species exhibited strong antioxidant and anti-bacterial activities due to the presence of sulfated polysaccharides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Enteromorpha species (Enteromorpha sp.) are edible seaweed that can grow abundantly in littoral zones of polluted and eutrophicated coastal marine waters. A green alga, Enteromorpha, has been used as a food diet in East Asia. It is grown and cultivated along seashores throughout the world, most notably in Japan, Korea, and China [1]. Another ancient report says that Enteromorpha is not only used as food but also as a natural medicine for treating fever, epistaxis, inflammation, and hydrops fetalis [2]. The cell wall of filamentous green macroalgae contains sulfated polysaccharides [3, 4]. Enteromorpha contains abundant nutrients, such as polyunsaturated fatty acids, dietary fiber, vitamins, and minerals [5]. The phytochemicals in Enteromorpha were reported as carotenoids, chlorophyll, phycocyanin, phenolic compounds, and flavonoids [6]. Sulfated polysaccharide possesses antioxidants [7,8,9,10,11,12, 15], anti-cancer, anti-coagulant, anti-hyperlipidemic, and antiviral activities. [13]. The algal extract of sulfated polysaccharide showed in vitro action against COVID-19 (SARS-CoV-2) [14]. It is having a high industrial demand owing to its potential biological applications [15,16,17]. In a study conducted by Baek et al. [18], it was demonstrated that an ethyl acetate extract of Enteromorpha prolifera (EAEP) exhibited the strongest antioxidant activity. Interestingly, the chemical composition of sulfated polysaccharides and their activity depends on the geographic location [19, 20].
The antioxidant activity of the extracted sulfated polysaccharides (SPs) from red algae was studied by Souza and his research team [21]. The cell walls of marine algae are mainly composed of sulfated polysaccharides (SPs). The isolated SPs from red, brown, and green algae are classified into carrageenans, fucoidan, and ulvan respectively based on the chemical composition [22]. Chen et al. [23] isolated the SPs from Grateloupia filicina (red algae) and studied its anticoagulant property. The SPs in aqueous extract of Ulva americana (green algae) were evaluated to assess their antibacterial activity against gram-positive and negative bacterial species by Berri et al. [24]. The immune-modulatory activity of the derived SPs from the Enteromorpha prolifera was also reported in the literature [25, 26]. The extracted SPs from Janiaruhens (red algae) and Ulva lactuca (green algae) were evaluated to analyze their antioxidant and phenolic contents by Essa et al. [27]. The promising biological applications of the polysaccharides were extensively reviewed by Vavilala et al. [28] and Chunyan and his coworkers [29]. The immunological activity of extracted SPs from Caulerpa lentillifera (green algae) was studied by Zhang et al. [30]. The study of Wang and his coworkers [31] reported that the aqueous extract of Enteromorpha linza (green algae) showed excellent anticoagulant properties due to the presence of SPs in the extract. The antimicrobial potential of extracted SPs from Chaetomorpha linum (green algae) was evaluated by Hamzaoui et al. [32]. The various marine algae such as Caulerpa lentillifera [33], Enteromorpha intestinalis [34], Codium divaricatum [35], Caulerpa racemosa var. peltata [36], Caulerpa sertularioides [37], and Caulerpa racemosa [38] were also evaluated for their wide range of biological activities. The SPs were also extracted from Lithothamnion muelleri (red algae) to study its antiherpes activity by Malagoli et al. [39]. Salehi et al. reported that Anacardium exhibited antioxidant, antimicrobial, and anticancer effects [40]. Similarly, another study conducted in Bangladesh proved that the presence of bioactive phytochemicals like phenols, tannins, and flavonoids in Amaranthus lividus and Amaranthus hybridus species exhibited strong antioxidant, anticancer, and antimicrobial activities [41]. This study intends to analyze the biological activities of SPs in the aqueous extract of Enteromorpha species (green algae) against pathogenic strains. Covelong (Latitude12.7898° N, Longitude 80.2542° E) is the unexplored marine hotspot for the isolation of new and potential aquatic organisms [42, 43]. Hence, the current study was planned to isolate the Enteromorpha sp. from the Covelong seashore area, and its crude extract was also screened for antioxidant and antibacterial properties.
2 Materials and methods
2.1 Collection of marine algae
The green algae of Enteromorpha sp. were collected and stored in a sterile glass bottle. The collected algae sample was identified by a standard seaweed manual [44]. From the sample, algal epiphytes and necrotic parts were removed by rinsing them with sterile water. After rinsing, Enteromorpha sp. was shade dried at room temperature for 5 days before the extraction process.
2.2 Crude extract preparation
Enteromorpha sp. powder was subjected to a cold acidic extraction. In brief, the sample was defatted and decolorized in methanol:acetone solvent mixture (3:7) followed by 2-day stirring in 1 N HCl. An equal volume of ethanol was added to the final extract (Fig. 1). This ethanol suspension was left overnight at − 20 °C. The precipitate was separated by centrifugation (3840 g for 60 min at 4 °C) and stored at 4 °C. This extract was further subjected for bio-chemical analysis and screened for antioxidant, anti-bacterial, and cytotoxicity effects.
2.2.1 Biochemical analysis
The presence of sulfate, total carbohydrates, xylose and protein was identified in the Enteromorpha sp., extract. In brief, the total carbohydrate level was estimated by the phenol–sulfuric acid method [45]. The barium chloride–gelatin protocol was used to determine the sulfate content, and the potassium sulfate was used as standard. The monosaccharide xylose was estimated using the orcinol method [46]. The protein level was estimated by Lowry’s method [47].
2.3 Phytochemical test
The fresh extract of Enteromorpha sp. was examined to analyze the presence of phytochemicals such as flavonoid and phenolic compounds. The method of analysis is discussed here.
2.4 Flavonoids
An alkaline reagent test was used to determine the presence of flavonoids in algal crude extracts. Ten milligrams of aqueous crude extract was mixed with 3 ml of 2% sodium hydroxide solution to carry out this analysis. The presence of flavonoids in the extract was identified by the formation of intense yellow color (Fig. 2a).
2.5 Phenolic compounds
Lead acetate test was used to identify the presence of phenols in algal crude extracts. In this study, the crude ethanolic extract of Enteromorpha sp. produced a bulky white precipitate (Fig. 2b) upon the addition of 10% lead acetate solution. This result confirms the presence of phenolic compounds in the extract of Enteromorpha sp.
2.6 In vitro antioxidant activities
In vitro antioxidant activity of Enteromorpha sp. was proved by determining the total antioxidant capacity and free radical scavenging activity. The determination of total antioxidant potential was done by phosphomolybdenum assay [48]. The assay principle involves the reduction of Mo (VI) to produce a green complex at lower pH conditions. One hundred microliters, 200 µl, 300 µl and 400 µl of algal extracts were mixed with 1 ml of DMSO and incubated in a water bath at 95 °C for 90 min. After incubation, the absorbance values of the mixture were read at 695 nm. The ascorbic acid (10 mg/ml DMSO) was used as a standard. The % phosphomolybdenum reduction potential (PRP) was calculated by the following formula:
where Abs (control) is the absorbance value of the control and Abs (sample) is the absorbance value of the extracts.
2.7 Free radical scavenging determination
The DPPH free radical–scavenging assay was carried out to examine the antioxidant potential of the crude extract of Enteromorpha sp. [49]. This assay may prevent the oxidation of the substrate. The experiment was carried out with the addition 5 μl, 10 μl, 15 μl, and 20 μl of algal extract to the mixture of 40 μl DMSO and 2.96 ml of DPPH (0.1 mM). The reaction mixture was then incubated under dark condition for 20 min at room temperature to record the readings at 517 nm. Three milliliters of DPPH was used as a control.
where Abs (control) is the absorbance value of the control, and Abs (sample) is the absorbance value of the extracts/standard.
2.8 In vitro antibacterial activity
The agar disc diffusion method was used to determine the antibacterial efficacy. One hundred microliter, 150 µl, and 200 µl concentrations of Enteromorpha sp. extracts were saturated in the sterile paper disc and tested against Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli. After 48 h of incubation, the inhibitory zones were measured. The agar diffusion method was done according to the guidelines of the Clinical and Laboratory Standard Institute (CLSI) [50, 51].
3 Results and discussion
The extract of Enteromorpha sp. was investigated to analyze its antioxidant, antibacterial, and anticancer properties. All the obtained results were compared and discussed critically to conclude the antioxidant and antibacterial potentials of SPs.
3.1 Chemical analysis of sulfated polysaccharides
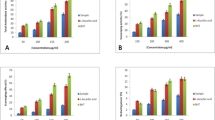
The sulfated polysaccharides of Enteromorpha sp. are an excellent source of sulfate and xylose [52]. In this current study, the extract of Enteromorpha sp. was quantitatively examined for carbohydrate, sulfate, xylose, and proteins, respectively. The existence of these essential ingredients was determined at a significant level in the extract. They are as follows: carbohydrate up to 0.13 g/ml (phenol–sulfuric acid method) (Fig. 3a and c); sulfate up to 0.0153 g/ml (Barium chloride-gelatin method) (Fig. 3b and d); xylose up to 0.0819 g/ml (Orcinol method) (Fig. 4a and c), and protein up to 0.0363 g/ml (Lowry’s method) (Fig. 4b and d). This analysis was highly correlated with the previous studies on Corallina officinalis, Pterocladia capillacea [53], Laminaria japonica [54], and Fucus vesiculosus [55]. Hence, it confirms the presence of sulfated polysaccharides in the extract.
3.2 Antioxidant activity of extract of Enteromorpha spices
The antioxidant efficacy of Enteromorpha sp. was examined by performing total antioxidant and DPPH-free radical scavenging assays. SPs derived from marine seeds are known for antioxidant activity [56]. In both the assays, ascorbic acid was used as a standard. In the total antioxidant assay, the scavenging potential of the extract of Enteromorpha sp. was determined. The Enteromorpha sp., extract effectively reduced the molybdenum [Mo (IV)] into phosphomolybdenum which paved the way for the formation of dark green color (Fig. 5a). It was confirmed with the development of a dark green color appearance. The extract of Enteromorpha sp. showed a free radical scavenging effect of 23 ± 0.35, 41 ± 0.05, 60 ± 0.52, and 81 ± 0.5% at the various extract concentrations of 100 μL, 200 μL, 300 μL, and 400 μL respectively (Fig. 5c). This result proved that Mo (IV) reducing activity is dose-dependent. Sulfated polysaccharides present in the Enteromorpha sp. may act as the reducing agents to scavenge the free radicals [57]. But the activity is higher compared to the studies done with other algal extracts [58].
3.3 DPPH-free radical scavenging assay
During the DPPH assay, the free radical scavenging activity was confirmed by the formation of yellow color (Fig. 5b). At the algal extract concentrations of 5, 10, 15, and 20 μg mL−1, the free radical scavenging capacity was measured as 69 ± 0.52, 75 ± 0.34, 79 ± 0.82, and 87 ± 0.82% respectively (Fig. 5d). When the concentration was increased from 5 to 20 μg mL−1, the scavenging capability also increased. Hence, the scavenging activity is dose-dependent for the prepared extract of Enteromorpha sp. It was observed that 20 μg mL−1 showed the highest percentage of activity. The obtained results of algal extract were compared with the ascorbic acid standard. The current results are in good agreement with a previous study [59, 60].
3.4 Antibacterial determination
The extract of Enteromorpha sp. had been tested against P. aeruginosa, S. aureus, and E. coli to analyze its antibacterial activity by disc diffusion method (Fig. 6). At the concentration of 150 mL−1 and 200 mL−1, it produced a zone of clearance with the diameter of 11 ± 0.2 mm and 13 ± 0.2 mm, respectively against Pseudomonas aeruginosa (Fig. 6a). It exhibited inhibitory zones of 10 ± 0.2 mm, 16 ± 0.2 mm, and 18 ± 0.2 mm at the concentration of 100 mL−1, 150 mL−1, and 200 mL−1, respectively for Staphylococcus aureus (Fig. 6b) and 11 ± 0.2 mm, 15 ± 0.2 mm, and 18 ± 0.2 mm respectively against Escherichia coli (Fig. 6c). The difference in the inhibitory zones is due to the resistance pattern of the organisms. The antibacterial activities of marine algal extract are mainly due to the presence of sulfated polysaccharides [61]. However, it showed less activity against gram-negative bacteria [62]. The results of the disc diffusion assay proved the broad-spectrum antibacterial efficacy of Entermorpha sp. Srikonga et al. studied the effects of green seaweed, U. intestinalis. The algal extract demonstrated antimicrobial activity against gram-positive bacteria, with inhibition zones ranging from 6.85 ± 0.17 to 16.4 ± 2.4 mm [63]. U. intestinalis was also found to possess strong antioxidant activity. It was reported that the methanolic extract of U. intestinalis exhibited the highest DPPH scavenging activity (48% inhibition) and a lower IC50 value of 2.32 mg/ml [64]. Kim and Jeong [65] investigated the antimicrobial and antioxidant activities of Enteromorpha intestinalis. Three solvents were used by them to obtain the extracts of Enteromorpha intestinalis. The obtained results proved that the extracts exhibited strong antimicrobial and antioxidant activity [65].
4 Conclusion
The extract of Enteromorpha sp. showed significant biological activities. The presence of major constituents in the extract of Enteromorpha sp. was identified as sulfate, xylose, carbohydrate, and proteins. Phytochemicals like phenolic compounds and flavonoids were also present in the marine algal extract. The radical scavenging activity of the Enteromorpha sp. was found to be increased from 23 ± 0.35 to 81 ± 0.5% with the increasing concentration of the algal extract. Therefore, the radical scavenging activity of Enteromorpha sp. was concluded as dose-dependent. The diameter of the inhibitory zone was increased from 10 ± 0.2 to 18 ± 0.2 mm for Staphylococcus aureus and from 11 ± 0.2 to 18 ± 0.2 mm for Escherichia coli while increasing the concentration of algal extract from 100 to 200 mL−1. This demonstrated the excellent antioxidant and antibacterial properties of the Enteromorpha sp. The extract of Enteromorpha sp. may be used for the betterment of mankind owing to its promising biological activities.
Abbreviations
- MTT-3:
-
(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- MCF-7:
-
Michigan Cancer Foundation-7 (human breast cancer cell line)
- COVID-19:
-
Coronavirus disease 19
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- DMSO:
-
Dimethyl sulfoxide
References
Yan X, Yang C, Lin G, Chen Y, Miao S, Liu B, Zhao C (2018) Antidiabetic potential of green seaweed Enteromorpha prolifera flavonoids regulating insulin signaling pathway and gut microbiota in type 2 diabetic mice. J Food Sci 84:165–173
Wei J, Wang S, Liu G, Pei D, Liu Y, Liu Y, Di D (2014) Polysaccharides from Enteromorpha prolifera enhance the immunity of normal mice. Int J Biol Macromol 64:1–5
Joel TK, Marie M, Rockyde N, Christopher RKG (2019) Ulvan: a systematic review of extraction, composition and function. Algal Res 39:101422
Ciancia M, Fernández PV, Leliaert F (2020) Diversity of sulfated polysaccharides from cell walls of coenocytic green algae and their structural relationships in view of green algal evolution. Front Plant Sci 11:554585
Baek SY, Kim MR (2019) Comparison of quality characteristic and antioxidant activity of Enteromorpha prolifera from Seosan and Muan in Korea. J Korean Soc Food Sci Nutr 48:1070–1078
Lin G, Liu X, Yan X, Liu D, Yang C, Liu B, Huang Y, Zhao C (2019) Role of green macroalgae Enteromorpha prolifera polyphenols in the modulation of gene expression and intestinal microflora profiles in type 2 diabetic mice. Int J Mol Sci 2:25
Gupta S, Abu-Ghannam N (2011) Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov Food SciEmergTechnol 12:600–609
Finosh GT, Jayabalan M (2013) Hybrid amphiphilic bimodal hydrogels having mechanical and biological recognition characteristics for cardiac tissue engineering. RSC Adv 5:38183–38201
Jimenez-Estrada M, Velazquez-Contreras C, Garibay-Escobar A, Sierras-Canchola D, Lapizco-Vazquez R, Ortiz-Sandoval C (2013) In vitro antioxidant and antiproliferative activities of plants of the ethnopharmacopeia from Northwest of Mexico. BMC Complement Altern Med 13:12
Litescu SC, Radu GL (2011) Bienzymatic sensor based on the use of redox enzymes and chitosan–MWCNT nanocomposite. Evaluation of total phenolic content in plant extracts. MicrochimicaActa 172:177–184
Teketay W, Xinyi D, Chunyan X, Ruxia W, Xin W (2022) Dietary Enteromorpha polysaccharide-Zn supplementation regulates amino acid and fatty acid metabolism by improving the antioxidant activity in chicken. J Anim Sci Biotechnol 13(1):18
Wassie Teketay, Zhuang Lu, Duan Xinyi, Xie Chunyan, Gebeyew Kefyalew, Yumei Zhang, Yin Yulong, Xin Wu (2021) Dietary Enteromorpha polysaccharide enhances intestinal immune response, integrity, and caecal microbial activity of broiler chickens. Front. Nutr 29:8. https://doi.org/10.3389/fnut.2021.783819
Kidgell JT, Magnusson M, deNys Rocky, Glasson CRK (2019) Ulvan: a systematic review of extraction, composition and function. Algal Res 39:101422
Kwon PS, Oh H, Kwon SJ et al (2020) Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov 6:50
Aguilera J, Dummermuth A, Karsten U, Schriek R, Wiencke C (2002) Enzymatic defences against photooxidative stress induced by ultraviolet radiation in Arctic marine macroalgae. Polar Biol 25(6):432–441
Andryukov BG, Besednova NN, Kuznetsova TA, Zaporozhets TS, Ermakova SP, Zvyagintseva TN, Chingizova EA, Gazha AK, Smolina TP (2020) Sulfated polysaccharides from marine algae as a basis of modern biotechnologies for creating wound dressings: current achievements and future prospects. Biomed 8:301
Nidhi H, Anushree M, Satyanarayan N (2021) Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: mini review. Bioresource Technol Reports 13:100623
Baek SY, Li FY, Kim DH, Kim SJ (2020) Enteromorpha prolifera extract improves memory in scopolamine treated mice via downregulating amyloid-β expression and upregulating BDNF/TrkB Pathway. Antioxidants 9(7):620–636
Siriluck I, Khanok R, Natta L, Chakrit T, Patthra P, Rattiya W (2016) Biochemical characteristics and antioxidant activity of crude and purified sulfated polysaccharides from Gracilariafisheri. Biosci, Biotech, and Biochem 80(3):524–532
Teketay W, Kaimin N, Chunyan X, Haihua W, Wu X (2021) Extraction techniques, biological activities and health benefits of marine algae Enteromorpha prolifera polysaccharide. Front. Nutr, 07 October 2021. https://doi.org/10.3389/fnut.2021.747928
Souza BWS, Cerqueira MA, Bourbon AI, Pinheiro AC, Martins JT, Teixeira JA, Coimbra MA, Vicente AA (2012) Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed Gracilariabirdiae. Food Hydrocol 27:287–292
Manlusoc JKT, Hsieh C-L, Hsieh C-Y, Salac ESN, Lee Y-T, Tsai P-W (2019) Pharmacologic application potentials of sulfated polysaccharide from marine algae. Polymers 11(7):1163
Chen X, Yang S, Wang J, Song L, Xing R, Liu S, Yu H, Li P (2015) Sulfated polysaccharides isolated from cloned Grateloupia filicina and their anticoagulant activity. Biomed Res Int 2015:612352
Berri M, Slugocki C, Olivier M et al (2016) Marine-sulfated polysaccharides extract of Ulvaarmoricana green algae exhibits an antimicrobial activity and stimulates cytokine expression by intestinal epithelial cells. J ApplPhycol 28:2999–3008
Kim JK, Cho ML, Karnjanapratum S, Shin IS, You SG (2011) In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorphaprolifera. Int J Biologic Macromolecules 49:1051–1058
Yumei Z, Xinyi D, Teketay W, Hai-hua W, Tiejun L, Chunyan X, Xin W (2022) Enteromorpha prolifera polysaccharide–zinc complex modulates the immune response and alleviates LPS-induced intestinal inflammation via inhibiting the TLR4/NF-κB signaling pathway. Food Funct. https://doi.org/10.1039/D1FO02171K
Essa HL, Guirguis HA, El-Sayed MH, Rifaat D, Abdelfattah MS (2020) Ultrasonically-extracted marine polysaccharides as potential green antioxidant alternatives. Proceedings 67:23
Vavilala SL, D´Souza JS, (2015) Algal polysaccharides and their biological applications, in marine algae extracts: processes, products, and applications. John Wiley & Sons Hoboken, New Jersey
Chunyan X, Yumei Z, Kaimin N, Xiaoxiao L, Haihua W, Junwei S, Xin W (2021) Enteromorpha polysaccharide-zinc replacing prophylactic antibiotics contributes to improving gut health of weaned piglets. Animal Nutrit 7(3):641–649
Zhang M, Meihui Z, Yudie Q, Yuanyuan L, Guanghua X, Yongcheng L (2020) Study on immunostimulatory activity and extraction process optimization of polysaccharides from Caulerpalentillifera. Int J BiologMacromol 143:677–684
Xiaomei W, Zhongshan Z, Zhiyun Y, Mingxing Z, Huimin Q (2013) Sulfation, anticoagulant and antioxidant activities of polysaccharide from green algae Enteromorphalinza. Int J BiologMacromol 58:225–230
Hamzaoui A, Ghariani M, Sellem I, Hamdi M, Feki A, Jaballi I, Nasri M, Amara IB (2020) Extraction, characterization and biological properties of polysaccharide derived from green seaweed “Chaetomorphalinum” and its potential application in Tunisian beef sausages. Int J BiolMacromol 1(148):1156–1168
Sun Y, Liu Z, Song S, Zhu B, Zhao L, Jiang J, Liu N, Wang J, Chen X (2020) Anti-inflammatory activity and structural identification of a sulfated polysaccharide CLGP4 from Caulerpalentillifera. Int J BiolMacromol 146:931–938
Li X, Xiong F, Liu Y, Liu F, Hao Z, Chen H (2018) Total fractionation and characterization of the water-soluble polysaccharides isolated from Enteromorphaintestinalis. Int J BiolMacromol 111:319–325
Li N, Mao W, Yan M, Liu X, Xia Z, Wang S, Xiao B, Chen C, Zhang L, Cao S (2015) Structural characterization and anticoagulant activity of a sulfated polysaccharide from the green alga Codiumdivaricatum. CarbohydrPolym 121:175–182
Hao H, Han Y, Yang L, Hu L, Duan X, Yang X, Huang R (2019) Structural characterization and immunostimulatory activity of a novel polysaccharide from green alga Caulerparacemosavarpeltata. Int J BiolMacromol 134:891–900
Chaves F, de Sousa AFG, Viana RLS, Rocha HAOSR, de Batistuzzo M, Moreira SMG (2019) Osteogenic activity of non-genotoxic sulfated polysaccharides from the green seaweed Caulerpasertularioides. Algal Res 42:101546
Ribeiro NA, Chaves HV, da ConceiçãoRivanor RL, do Val DR, de Assis EL, Silveira FD, Gomes FIF, Freitas HC, Vieira LV, da Silva Costa DV, de Castro Brito GA, Bezerra MM, Benevides NMB (2020) Sulfated polysaccharide from the green marine algae Caulerparacemosa reduces experimental pain in the rat temporomandibular joint. Int J BiolMacromol 150:253–260
Malagoli BG, Cardozo FTGS, Gomes JHS, Ferraz VP, Simões CMO (2014) Chemical characterization and antiherpes activity of sulfated polysaccharides from Lithothamnionmuelleri. Int J BiologMacromol 66:332–337
Salehi B, Ozguven MG, Kirkin C, Ozcelik B, Marais Braga M, Carnerio JNP, Bezerra CF, Silva TG, Coutinho HDM, Amina B, Armstrong L, Selamoglu Z, Sevindik M, Yousaf Z, Sharifi-Rad J, Muddathir AM, Devkota HP, Martorell M, Jugran AK, Cho WC, Martins N (2020) Antioxidant, antimicrobial, and anticancer effects of anacardium plants: an ethnopharmacological perspective. Front Endocrinol 11:295
Mamun MA, Husna J, Khatun M, Hasan R, Kamruzzaman M, Hoque KMF, Abu RZ, Ferdousi, (2016) Assessment of antioxidant, anticancer and antimicrobial activity of two vegetable species of Amaranthus in Bangladesh. BMC Complement Altern Med 16:57
Alexei VC, Neonila EP, Mohandhas SV, Ruchi PJ, Prakash S, Jon LN, Jon LN, Rajaian PR (2020) A histology-free description of a new species of the genus Tetrastemma (Nemertea: Hoplonemertea: Monostilifera) from Hawaii and India. Zootaxa 4808(2):379–383
Chandan J, Aman S, Sivakumar J, Malaiyarasa P (2013) Surface associated bacteria of marine algae in kovalam beach, Chennai, had screened for its antifouling activity. Ind J Geo-Mar Sci 42(4):498–502
Dhargalkar (2004) Seaweeds—a field manual. National Institute of Oceanography, Dona Paula, Goa. 403004
Albalasmeh AA, Berhe AA, Ghezzehei TA (2013) A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. CarbohydrPolym 97(2):253–261
Blackmore PF, Williams JF, MacLeod JK (1976) FEBS Lett 64:222–226
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J BiolChem 193:265–275
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthone on the auto oxidation of soybean in cylcodextrin emulsion. J Agri Food Chem 40:945–948
Humphries RM, Ambler J, Mitchell SL et al (2018) CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J Clin Microbiol 56(4):e01934-e2017
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Guangling J, Guangli Y, Junzeng Z, Stephen E (2011) Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs 9(2):196–223
Mona MI, Mohamed SA (2020) Characterization and biological properties of sulfated polysaccharides of Corallinaofficinalis and Pterocladiacapillace. Acta Bot Bras 34(4):623–632
Xue CH, Fang Y, Lin H et al (2001) Chemical characters and antioxidative properties of sulfated polysaccharides from Laminariajaponica. J Appl Phycol 13:67–70
Rodriguez-Jasso RM, Mussatto SI, Pastrana L et al (2014) Chemical composition and antioxidant activity of sulphated polysaccharides extracted from Fucusvesiculosus using different hydrothermal processes. Chem Pap 68:203–209
Olasehinde TA, Olaniran AO, Okoh AI (2020) Sulfated polysaccharides of some seaweeds exhibit neuroprotection via mitigation of oxidative stress, cholinergic dysfunction and inhibition of Zn-induced neuronal damage in HT-22 cells. BMC Complement Med Ther 20:251
Duan XJ, Zhang WW, Li XM, Wang BG (2006) Evaluation of antioxidant property of extract and fractions obtained from a red alga. Polysiphoniaurceolata Food Chem 95:37–43
Poliana O, Glauber C, Francisco N, Costa LEC, Carla V, Willer MG, Clara M, Ewerton S, Edivânia AC, Regina C, Ana L (2019) A novel antioxidant sulfated polysaccharide from the algae Gracilariacaudata: in vitro and in vivo activities. Food Hydrocolloid 90:28–34
Khan BM, Zheng L-X, Khan W, Shah AA, Liu Y, Cheong K-L (2021) Antioxidant potential of physicochemically characterized Gracilariablodgettii sulfated polysaccharides. Polymer 13:442
Si-Min Q, Jude Juventus A, Xiaojuan L, Yang L, Shijie T, Wancong Z, Kit-Leong C (2022) Bioactive polysaccharides from red seaweed as potent food supplements: a systematic review of their extraction, purification, and biological activities. Carb Pol 275:118696
Rodrigo das N, José A, Márjory L, Ana L, Regina C, Vânia M, Norma M (2012) Antimicrobial effect of a crude sulfated polysaccharide from the red seaweed Gracilariaornata. BrazArch BiolTechnol 55(2):171–181
Joon-Young J, Min-Jeong J, In-Hak J, Koji Y, Yuji K, Byoung-Mok K (2018) Antimicrobial and antibiofilm activities of sulfated polysaccharides from marine algae against dental plaque bacteria. Mar Drug 16(9):301
Srikonga W, Bovornreungrojb N, Mittraparparthorna P, Bovornreungroja P (2017) Antibacterial and antioxidant activities of differential solvent extractions from the green seaweed Ulva intestinalis. Science Asia 43:88–95
Farasat M, Khavari-Nejad RA, Nabavi SMB, Namjooyan F (2014) Antioxidant activity, total phenolics and flavonoid contents of some edible green seaweeds from northern coasts of the Persian Gulf. Iran J Pharmaceut Res 13:163–170
Kim DH, Jeong GT (2014) Antimicrobial and antioxidant activities of extracts of marine green-algae Enteromorpha intestinalis. Korean Soc Biotechnol Bioeng J 29:92–97
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Swathi, N., Kumar, A.G., Parthasarathy, V. et al. Isolation of Enteromorpha species and analyzing its crude extract for the determination of in vitro antioxidant and antibacterial activities. Biomass Conv. Bioref. 14, 3753–3762 (2024). https://doi.org/10.1007/s13399-022-02591-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02591-1