Abstract
We present a study of habitat use, oviposition plant choice, and food plant suitability for the checkerspot butterfly Melitaea athalia Rottemburg (Lepidoptera: Nymphalidae) in Åland, Finland. We found that in Åland, unlike in the mainland of Finland and many parts of its range, M. athalia flies mainly in open meadows. When offered an array of plants in a large (32 × 26 m) field cage, they predominately oviposited upon Veronica chamaedrys L., V. spicata L. and Plantago lanceolata L. (Plantaginaceae), which grow in open meadows. The relative abundance of the butterfly in Åland, and its habitat and host plant use there, may reflect local adaptation to land use practices and geology that maintain clusters of small open meadows with little successional change. At the scale of a plant patch, preferred species were used as frequently in mixed species patches as in mono-specific patches, and more oviposition occurred in open than in grassy patches. All of the host plants used by M. athalia are defended by iridoid glycosides (IGs). However, oviposition choice among species and among individual plants within species was largely independent of IG concentration. This contrast with the more discerning congener, M. cinxia, supports the idea that host discrimination decreases with increasing host range. Finally, although the adult butterflies chose specific plant species for oviposition, as larvae they performed well on twelve out of thirteen species of plants, including both known hosts and related novel plants that occur in Åland, indicating a much wider range of larval food plant species than adult oviposition species.
Similar content being viewed by others
Introduction
Most herbivorous insects are selective in their oviposition choice, both among plant species, and among plant individuals within these species (e.g., Ng 1988; Singer and Lee 2000). What criteria are used and how stringent they are defines the host range, as well as the habitats in which a herbivore is found. The checkerspot butterflies (the Melitaeine in the family Nymphalidae) are oligophagous or monophagous on plant species belonging to 16 families that inhabit meadows, forest edges, sparse forest and clearings (Ehrlich and Hanski 2004). Most of the plants used are in 11 families that share iridoids as plant secondary compounds (Jensen et al. 1975; Jensen 1991; Wahlberg 2001). The most common group of these iridoids are the iridoid glycosides (IGs) (Damtoft et al. 1997; Li et al. 1999; Sturm and Stuppner 2001), which are deterrent to many generalist herbivores (Bowers and Puttick 1986; Stamp 2001), but are tolerated or even sequestered by specialists (Mead et al. 1993; Stermitz et al. 1994; Suomi et al. 2001; Harvey et al. 2005). Sequestration of IGs is thought to make the caterpillars unpalatable or unsuitable for generalist vertebrate and invertebrate predators (Bowers 1980; Camara 1997).
In this study we investigate habitat and host plant use by the oligophagous Heath fritillary butterfly, Melitaea athalia (Rottemburg, 1775) (Lepidoptera: Nymphalidae) in the Åland islands, Southwestern Finland. Melitaea athalia is part of an ecologically and evolutionarily well-studied group of butterflies (Ehrlich and Hanski 2004) which are of interest to conservation ecologists (Hanski et al. 2004). The critical factor governing the survival of M. athalia is thought to be its dependence on short lived successional habitats (Warren 1987a, b, c). The species has declined severely in most of Europe, including Britain, Flanders, the Netherlands and Switzerland (Warren et al. 1984; Lepidopterologen-Arbeitsgruppe 1988; Brink 1992; Schwarzwälder et al. 1997; Gorissen et al. 2004), but it is a common species in southern Finland and Åland (Marttila et al. 1990; Wahlberg et al. 2002; Schulman et al. 2005). The aim of this paper is to describe habitat and host plant use by M. athalia in Åland where it is not in decline. A second motivation for this study is to assess the ecological overlap of M. athalia with the co-occurring Glanville fritillary butterfly, Melitaea cinxia (Linnaeus, 1758) (Lepidoptera: Nymphalidae) (Ehrlich and Hanski 2004). These butterflies share some host plants, and solitary M athalia larvae are often found inside M. cinxia larval groups. The species may interact indirectly through generalist (Pteromalus apum) and specialized (Ichneumon cinxiae and I. gracilicornis) pupal parasitoids (Shaw et al. in press).
First, we present the natural habitat use of the adult butterfly using transect counts during the flight season in potential M. athalia habitat. Because human land use in Åland is different from that in mainland Finland and Britain we expect the habitat use by M. athalia to differ. Second, we present an oviposition choice experiment in a large outdoor cage, in which butterflies could freely oviposit on potential host plant species over a 2-week period. The plant species available to the butterfly included most of the species that are known to be fed upon by M. athalia larvae as well as several related species present in Åland (Table 1). For the congener M. cinxia, iridoid glycosides are known to be positively associated with oviposition choice (Nieminen et al. 2003; Reudler Talsma et al. in press) and larval development (Harvey et al. 2005; Saastamoinen et al. 2007). To investigate the role of IGs in M. athalia host plant choice, both with respect to their discrimination among species and among individuals, we used HPLC to analyze the aucubin and catalpol (IGs) content of all plants in the oviposition experiment. Finally, we reared prediapause larvae on 13 different potential food plants. These plants included the species used in the oviposition experiment as well as five related species growing in habitats that could potentially be used by M. athalia in Åland. This allowed us to compare adult oviposition preference with performance of the larvae (development time, survival and diapause weight).
Material and methods
Natural history and study system
Melitaea athalia is vulnerable to habitat loss because it is relatively sedentary, and depends on a well connected network of habitat patches that are often successional (Warren 1987a; Wahlberg et al. 2002; Franzen and Ranius 2004). In Britain, where M. athalia is endangered and has been well studied, it inhabits Plantago-rich grasslands, Melampyrum-rich woodland clearings and sheltered heathlands containing scattered Melampyrum (Warren 1987b). The larvae have been observed feeding on Plantago lanceolata L., P. major L., Veronica chamaedrys L., V. hederifolia L., V. serpyllifolia L., Digitalis purpurea L. (Plantaginaceae) and Melampyrum pratense L. (Orobanchaceae) (Warren 1987c). The major factor causing the decline of M. athalia in Britain during the last 150–200 years is thought to be the decline of chopping as woodland management, which has lead to a decrease of habitat for M. athalia (Warren 1987a, b). The same is true for the species in Flanders (Gorissen et al. 2004) and most of the Netherlands (Brink 1992). Similarly, in Switzerland M. athalia has declined with changing land use and is now found only locally in steep sparse mountainside grassland, grazed forest meadows, forest roadsides, dry limestone meadows and bog edges (Lepidopterologen-Arbeitsgruppe 1988).
In the mainland of Finland M. athalia is known to occur along forest edges and in openings within forests, such as wood clearings and abandoned fields (Selonen 1997; Wahlberg 1997; Wahlberg et al. 2002). Prediapause larvae have been recorded on V. chamaedrys, V. spicata, and Plantago lanceolata. Postdiapause larvae also feed on Melampyrum pratense. Females were observed to land and tap on V. chamaedrys and M. sylvaticum before ovipositing on an adjacent nonhost plant (Wahlberg 1997, 2000). In Åland eggs and post diapause larvae of M. athalia have been found on V. spicata, V. chamaedrys, and P. lanceolata (S. van Nouhuys, personal observation).
Habitat use during adult flying season
To compare the types of habitats used by adult M. athalia in Åland we conducted a transect study using the method of Pollard (1997). Melitaea athalia was generally common in the region chosen for the three transects, and the habitat was heterogeneous. The three 2.6–2.9 km transects were each divided into six sections according to habitat type. (1) Dry meadows: open sunny areas with small junipers and in some cases small pines, rocky outcrops and scattered flowering plans. (2) Mesic meadows and herb-rich road verges: sunny areas with many flowering plants such as Ranunculus spp., and Trifolium spp. (3) Semi-open forest and forest edges: mixed birch, spruce and pine, with shade and few flowering plants. (4) Field edges: high grass and few flowers in sunny open areas, exposed to wind. (5) Forest: cultivated pine with sun and shade and few flowering plants. (6) Herb-poor road verges: open sun with high grass.
The occurrence of M. athalia was monitored by one person walking each transect route twice a week for 6 weeks (weeks 23–28, 2005), on clear days between 11.00 and 17.30 h. All M. athalia butterflies seen within 5 m of the transect walker were counted. Each butterfly was caught to ensure correct identification and to record its sex. The behaviour of the butterflies prior of being caught was also recorded (feeding, mating, flying or basking).
Melitaea athalia used for experiments on oviposition choice and larval performance
The M. athalia butterflies and larvae used in the experiments were the progeny of field collected post diapause larvae from Åland that were reared to adulthood in the laboratory on a mixture of V. spicata and P. lanceolata leaves. Multiple males and females from approximately twenty collection sites were mated and the females were placed outside in sleeve cages with potted V. chamaedrys and V. spicata for oviposition. The eggs were collected daily and moved to Petri dishes. Upon hatching the larvae to be used as adult butterflies were fed V. spicata and P. lanceolata leaves throughout their development. The larvae used for the larval feeding experiment were moved to their experimental plant soon after hatching (see larval performance).
Oviposition host plant choice
This experiment was conducted in 2005 in a large cage (26 × 30 × 3 m) covered with a mesh cloth that allowed natural environmental conditions inside. The natural vegetation in the cage included nectar plants that the butterflies could feed upon, but no potential host plants. The cage was previously used to measure the movement behaviour and life history traits (longevity and fecundity) of adult M. cinxia (Saastamoinen 2007).
Six plant species were transplanted in the spring from natural populations in Åland into 9 cm diameter pots. Four species, P. lanceolata (Pl), V. spicata (Vs), V. chamaedrys (Vc), M. pratense (Mp), are known host plants of M. athalia. Two others, P. major (Pm) and V. officinalis (Vo) L. (Plantaginaceae) were included as potential host plants (Table 1). Each species was replicated 24 times for a total of 144 plants. Initially, two other plant species, M. sylvaticum (Ms) L. and M. nemorosum (Mn) L. (Orobanchaceae), were included in the experiment, but the conditions in most of the cage were too dry. Instead, ten individuals of each species were placed in a shady moist corner of the cage to see if M. athalia would use them. They were not included in the statistical analyses.
The 144 plants were set up in the cage in 12 groups of 12 plants each, in a three by four grid (Fig. 1). The distance between the pots in each group was 15 cm and the distance between the groups was 8 m. Six of the groups were monocultures of each species and the other six groups contained two plants of each of the six species.
Schematic drawing of the locations of plant groups in the cage, and the number of Melitaea athalia egg clusters laid in each location. Plots A, B, C, E, F and K are in bare patches (outlined plots), plots D, G, H, I, J and L are in grassy vegetation (no outline). Note that locations of the groups of plants were randomized daily so the habitat types are not associated with a plant species or configuration
The vegetation in the cage was not uniform. Plots A, B, C, E, F and K were bare areas, whereas, plots D, G, H, I, J and L were surrounded by vegetation (Fig. 1). To separate the effects of microclimate and neighbouring plants from plant species, the locations of the plants were rearranged every evening. Each of the 12 groups stayed together but the location of the group in the cage, as well as the order of the pots within each group was randomized.
An unrelated experiment was running in the cage simultaneously with this one (van Nouhuys and Kaartinen 2008), which meant that there were another 42 potted V. spicata plants available for oviposition throughout the experiment. These additional plants were subdivided in two groups: “transect” and “array”. The 20 transect plants were placed singly in a four by five grid, with 6 m between the plants. The 22 array plants were placed in two lines in the centre of the cage. The two lines where 18 m apart, and within each line the plants were spaced 5 cm apart. The extra V. spicata plants were at least 2 m away from any of the plots A–J.
Newly emerged adult M. athalia (93 females and 79 males) were individually marked and released into the cage between 1st of July and the 3rd of July. They were observed to begin feeding and mating soon after release. At the end of each day, between 17:30 and 19:00 h all of the plants (including the additional V. spicata plants) were checked for eggs. The egg clusters were collected and the number of eggs in each was counted. Occasionally multiple egg clusters were laid on a plant in a day. Neighbouring egg clusters could be distinguished by colour and pattern of distribution. The weather was extremely warm, and after 2 weeks (July 14th) most of the butterflies had died and the experiment ended.
Chemical analyses
To study the association between oviposition choice and plant secondary chemistry, we analysed the IGs aucubin and catalpol as the percentage of leaf dry weight for all the plants used in the oviposition experiment and the additional array and transect V. spicata plants. The iridoid glycosides aucubin and catalpol were analysed because they occur in high concentrations in these plant genera (Suomi et al. 2002 ) Furthermore they are associated with oviposition by M. cinxia (Nieminen et al. 2003) and Junonia coenia (Pereyra and Bowers 1988). Before the experiment started, one medium-aged leaf from each plant was taken and air-dried. The leaves were ground to a fine powder with a ball mill (type MM 301, Retsch GmbH & Co., Haan, Germany). Each 25 mg sample was extracted in 10 ml of 70% MeOH and shaken overnight. The crude extract was filtered on Whatman #4 filter paper and diluted ten times with Milli-Q water. The concentrations of aucubin and catalpol were analysed by HPLC using a Bio-LC (Dionex Corp., Sunnyvale, USA) equipped with a GP40 gradient pump, a Carbopac PA 1 guard (4 × 50 mm2) and analytical column (4 × 250 mm2). For Veronica species we used a Carbopac PA 20 guard (3 × 30 mm2); analytical column (3 × 150 mm2). For pulsed amperimetric detection (PAD) we used an ED50 electrochemical detector. NaOH (1M) and Milli-Q water were used as eluents (10:90%, 1ml/min). Retention times were 3.25 min and 4.40 min for aucubin and catalpol, respectively. Concentrations were analyzed using Chromeleon version 6.60 (Dionex Corp., Sunnyvale, USA).
Larval performance
Newly hatched M. athalia larvae were fed 13 different food plant species in Petri dishes in the laboratory. In addition to the eight food species used in the oviposition cage we included: Veronica longifolia L., Linaria vulgaris (Miller) (Plantaginaceae) Rhinanthus minor L., R. serotinus (Schönh) and Odontites littoralis (Fr.) (Orobanchaceae) (Table 1). All of these species occur in Åland in habitat that could potentially be used by M. athalia. First instar larvae from approximately 40 egg clusters were combined and then separated into 95 groups of 10 larvae (except those feeding on P. major and R. minor which were started in the second instar, when the host plants became available). The larvae were kept in groups because the early instars of M. athalia are gregarious. The groups were kept on filter paper in the Petri dishes, randomly assigned to feeding treatments and then fed leaves picked daily from naturally occurring plants. There were 10 replicate dishes of each of the five known food plants (except for M. nemorosum) as well as for V. longifolia and five replicates for the other (potential) food plants. We measured three performance parameters: (1) development time as the number of days from second instar until all of the larvae in a dish had reached diapause (4th instar), (2) the weight of individual larvae at diapause, and (3) the number of larvae in each dish surviving to diapause.
Statistical analysis
Statistical analyses for the oviposition experiment were performed using the statistical program SPSS v.13.0 (SPSS Inc., Chicago, Illinois). To test for differences in the number of egg clusters laid per plant species and per plot Kruskal–Wallis tests were used. A non-parametric test was used because the data were not normally distributed. Mann–Whitney U-tests were used for further pairwise comparisons among the plant species. We compared the number of egg clusters on plants in bare versus vegetated plots, and in the mixed versus single species groups using Mann–Withney U-tests as well. To test for differences in egg cluster sizes between the plant species we used analysis of variance with species, plant individual and mixed/single group as a factors. Within the mixed groups we analysed egg cluster size as a function of species, plant individual and group (replicate). Plant individual was nested in group × species. Egg cluster size was square root transformed to increase the normality of the distribution. These analyses did not include the transect and array V. spicata plant. To compare the number of egg clusters per V. spicata plant in the experimental plots A–J with those in the additional transect and array plants we used the Fisher’s exact test.
For the chemical analyses we used Statistica v 7.1 (StatSoft, Inc.). We performed an ANOVA on IG content with plant species and mixed versus single species groups as factors. A posthoc comparison (Tamhane) of IG content was used to distinguish among plant species. All of the plants in the cage were included in the analysis. Within the mixed groups we analysed IG content with species, plant individual and group as factors. To compare the IGs of plants with and without oviposition we used a t-test for each species separately. For each plant species we also calculated Pearson correlations to test for an association between the total numbers of egg clusters laid on a plant and the IG concentration of that plant, and between the average number of eggs in a cluster (total number of eggs on a plant divided by the number of clusters on that plant) and the IG concentration. The aucubin and total IG levels were log10 transformed and the catapol levels were square root transformed to increase the normality of their distributions.
The effect of food plant species on the performance of M. athalia larvae was analysed using analysis of variance in the statistical program Stata (Statacorp, College station, USA). For the analyses of development rate (number of days from second instar until all the larvae in a dish were in diapause) and survival (number of larvae surviving to diapause) the experimental unit was Petri dish, and the effect of plant species was tested. For the analysis of weight of individual larvae at diapause, Petri dish was nested within food plant species. Rhinanthus minor and P. major were not included in this analysis because these treatments were started later, when the larvae had already moulted to the third instar. For each of the three analyses (development rate, survival and weight at diapause), the plant species were compared by constructing post-hoc contrasts of the performance on each plant species with the average performance. Interpretations of the statistical differences were made using the Bonferroni adjustment for multiple comparisons.
Results
Habitat use
Altogether, 141 M. athalia butterflies were recorded during the 6 weeks of observation (ten transect walks), 118 males and 23 females. Most of the butterflies were flying (87♂; 7♀) at the moment of observation, the rest were on flowers (18♂; 7♀), basking (11♂; 7♀) or mating (2♂; 2♀). The butterflies on flowers were in both the dry (8) and mesic (17) meadows, on Ranunculus spp., Achillea millefolium, Allium schoenoprasum, Trifolium repens, Trifolium pratense, Leucanthemum vulgare, Filipendula ulmaria, Hieracium umbellatum and Knautia arvensis.
The density of M. athalia was highest in dry meadows, but they were also found in mesic meadows/herb-rich road verges, semi-open forest habitats/forest edges and field edges. The species was missing entirely from dense forests and from herb-poor open landscapes (Table 2).
Four of the known host plants were present, V. spicata (14.5% segments of the transects), V. chamaedrys (35.5%), P. lanceolata (32%) and M. pratense (6.5%). Butterflies were observed most frequently in transect sections where V. chamaedrys, V. spicata and P. lanceolata were abundant. That is, in 82%, 100% and 95%, of the sections of each transect where these plants were present, the butterfly was also found. In contrast, M. athalia was only present in 50% of the transect sections where M. pratense occurred.
Oviposition host plant choice
During the 2 weeks of the experiment M. athalia laid 455 egg clusters (on average 4.9 per female); 237 of them were on the actual study plants, one on M. nemorusum in the shady corner of the cage, and the other 217 on the V. spicata transect (55) and array (162) plants.
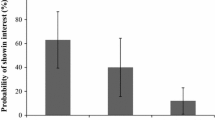
The number of egg clusters received by individual plants ranged form zero to 13 and differed significantly among plant species, with most egg clusters laid on V. chamaedrys, V. spicata and P. lanceolata (Kruskall-Wallis, P < 0.001; Fig. 2). This was the same in both the mixed and single species groups (Fig. 2; Mann–Whitney U-test P > 0.1 for each species). During the experiment, we observed that some females used more than one host plant species for ovipositing, even during the same day. Twelve individuals laid eggs on two different host plant species (eight oviposited on both V. chamaedrys and V. spicata, two on P. lanceolata and V. spicata, one on P. major and V. spicata, and one on V. spicata and V. officinalis).
The number of egg clusters laid on plants in single species (light grey bar) and mixed groups (dark grey bar). Transect and array V.spicata are not included here. Groups significantly different from each other in number of clusters (P < 0.05 using a Mann–Whitney U-test) are represented with different letters above the columns
Some areas in the cage attracted significantly more ovipositing females than others, regardless of which set of plants were present. The overall difference between the locations is statistically significant (Kruskall–Wallis test, P < 0.005) with bare plots (A, B, C, E, F and K) receiving more egg clusters (on average 28.8 cluster per plot) than vegetated plots (on average 10.7 clusters per plot) (D, G, H, I, J, L) (Fig. 1; Mann–Whitney U-test, P < 0.001).
Veronica spicata plants that were part of the arrays received significantly more egg clusters per plant (on average 3.7) than those in the transect (1.4 per plant) or in our main experimental V. spicata plants (1.6 per plant) (Fisher exact test array versus transect: P < 0.0001; array versus experiment plants: P < 0.02).
The mean egg cluster size in the experiment was 43.19 (s.e 1.9), with a minimum of three and a maximum of 201 eggs (this large cluster may have been from M. cinxia, accidentally brought into the cage on the plant). Overall, egg cluster size did not differ among plants species, nor between plants in mixed or pure stands. However, within the mixed plant groups we find a significant effect of species, with the largest clusters on M. pratense (ANOVA, F species = 2.41, P = 0.045; F group = 1.39, P > 0.1 and F plant = 1.20, P = 0.047, Fig. 3).
Sizes of Melitaea athalia egg clusters laid on each plant species. The median is indicated with the horizontal black bar in the box. The box encloses the upper and lower quartile and the error bars indicate the smallest and largest observations that were not outliers. The outliers are indicated with open circles, and the extreme with an asterisk
Iridoid glycoside content of plants in the oviposition experiment
The plant species differed in IG content (ANOVA, aucubin: F species = 57.1, P < 0.001; F mixed = 2.95, P > 0.1; catalpol: F species = 87.7, P < 0.01; F mixed = 0.001, P > 0.1; Fig. 4). The percent dry weight ranged from a low mean of 0.72 % in P. major up to 11.53% in M. pratense. The species that received most egg clusters (Vc, Vs, and Pl) had intermediate IG concentrations (Fig. 4).
Iridoid glycoside content (percentage per mg dry weight) of each plant species in the oviposition experiment. The circles and error bars are the means and standard errors for aucubin (black) and catalpol (white). The numbers at the top of each column are the number of plants analyzed. The letters adjacent to the circles indicate which plant species significantly differed in aucubin and catalpol content using a Tamhane posthoc test (letters for catalpol uppercase (left), and for aucubin lowercase (right))
Within species the IG concentration also varied among individuals (e.g., Vc had a minimum IG content of 0.29% and maximum content of 1.85%). For most plant species in the oviposition experiment there was no association of IG concentration with whether or not a plant received eggs, or the number of egg clusters it received. However, individual transect V. spicata plants that received egg clusters had higher levels of catalpol and total IG than plants that did not receive any eggs (t-test; catalpol: t = 2.53; df = 34; P < 0.02; total IG: t = 2.21; df = 34; P < 0.04). Similarly, for P. major and V. chamaedrys high total IG concentrations, and in particular high aucubin (for P. major) were correlated with a greater number of egg clusters on a plant (aucubin Pm: r = 0.5, n = 24, P < 0.01; total IG Pm: r = 0.49, n = 24, P < 0.01; Vc: r = 0.6, n = 12, P < 0.04).
Egg cluster size was not correlated with IG concentration except again for the transect V. spicata plants. In these plants the number of egg clusters ranged from zero to 12 and the average egg cluster size per plant ranged from 15.5 to 81 eggs, increasing in size with the catalpol concentration (r = 0.35, n = 36, P < 0.04 Fig. 5). There was a similar trend for total IG concentration (r = 0.31, n = 36, P < 0.07).
Larval performance
It took 12–21 days for all of the larvae in a dish to develop from second instar to diapause. Overall, food plant species was related to rate of development (ANOVA, F 10,79 = 10.99, P < 0.001). Larvae developed most quickly on M. nemorosum, and developed slowest on V. officinalis and M. sylvaticum (Fig. 6a).
The performance of prediapause Melitaea athalia larvae fed in the laboratory on six known host plants (grey bars) and seven potential food plants (black bars). (a) Rate of development (days from second instar to diapause); (b) survival: number of caterpillars surviving to diapause (out of 10 per dish); (c) mean weight at diapause. The letters on top of the bars indicate which plant species significantly differed from each other (P < 0.05; ANOVA with post hoc tests)
Few larvae died during the experiment, but there was a significant effect of food plant species on the per-Petri dish rate of survival (ANOVA, F 12,82 = 15.8, P < 0.001), with V. officinalis being particularly low (Fig. 6b). At diapause most larvae weighed between 4.5 and 5.5 mg. This differed significantly among those feeding on different food plants (ANOVA, F 12,669 = 6.28, P < 0.001), but only substantially for the small larvae feeding on V. officinalis (Fig. 6c).
Discussion
Habitat use
The observations of habitat use are mostly in agreement with a previous study done in Åland in the summer of 2002 in which Schulman et al. (2005) monitored the occurrence of several butterfly species in agricultural areas on randomly chosen plots of one square kilometre. On the mainland of Finland M. athalia has been found to use mostly semi-open forest habitats and to be absent from open meadows. Perhaps M. athalia was absent from these open meadows because the meadows are small and isolated (Selonen 1997), whereas in Åland open meadows are more common and less isolated because of differences in land use. In Britain habitat use by M. athalia is also known to differ regionally (Warren et al. 1984; Warren 1987c). For instance in south-western England abandoned hay meadows are the main habitat while in south-eastern England the species occurs along the margins of cleared plots next to deciduous forest (Warren 1984). This could be due to the distribution of suitable habitat in the landscape (van Nouhuys and Hanski 2002; Wahlberg et al. 2002; Bergman et al. 2004).
As discussed recently by Friberg et al. (2008), habitat availability may drive shifts in host plant use, or host plant preference per se can explain habitat use. In this case it is most plausible that because of the different land use in Åland the consistent availability of warm open meadows allows the use of meadow plants (P. lanceolata, V. chamaedrys and V. spicata) instead of forest edge and gap species (Melampyrum spp.).
Oviposition host plant choice
In the oviposition experiment M. athalia primarily laid eggs on V. chamaedrys, V. spicata and P. lanceolata, which occur mostly in meadows. This corresponds well to where we saw adults flying. Furthermore, we find eggs on these plants in natural populations in Åland. It is possible that this preference is not fixed, but due to larval conditioning (Jaenike 1983), that is, adults exhibit oviposition preferences corresponding to chemicals experienced during development (Barron 2001). If this were the case then the butterflies in our experiment may have used P. lanceolata and V. spicata at a higher than natural frequency because they were fed these plants as larvae.
There is also the possibility that V. spicata was used with high frequency because it was the most abundant plant in the cage, and the butterflies learned to seek it out. In this case we doubt that adult conditioning contributes significantly to the results because V. spicata was not used more in the experimental plots than V. chamaedrys or P. lanceolata. Further, while some species of butterflies have been shown to learn to search for common plant forms (Papaj and Rausher 1987), others, such as the closely related Euphydryas editha (Parmesan et al. 1995) and M. cinxia (Schöps and Hanski 2001) have been shown not to learn from previous oviposition experience.
In addition to our main set of 144 oviposition plants, the butterflies also had access to an extra 42 transect and array V. spicata plants. The array plants received a disproportionately high number of egg clusters. There are several possible explanations for this. First, the array plants were on bare ground which is preferred by the butterfly. Secondly, M. athalia females prefer to oviposit near the ground (Warren 1987c) and the array V. spicata pots were buried in the ground rather than placed on the soil which appeared to make them more accessible (van Nouhuys, personal observation).
Plant distribution and oviposition
Host plants in bare areas in the oviposition cage received three times more egg clusters than plants in vegetated plots. Warren (1987c) also found that M. athalia generally laid eggs on host plants in sparse vegetation. Plants that grow in a bare area may be more conspicuous than plants in vegetated areas and at least in meadows tend to occur in warm microclimates, which are more suitable for larval development. Some butterflies are known to preferentially lay eggs in host plant stands containing nectar flowers (Janz 2005; Janz et al. 2005b). This was not the case in our study, as the nectar plants occurred (and were used by the butterflies) in the vegetated areas of the cage, and the majority of the oviposition occurred in the open parts of the cage. Foraging for nectar does not appear to be at odds with foraging for oviposition plants, as found by Janz (2005) for the butterfly Vanessa cardui, perhaps because of the greater time investment in laying egg in clusters rather than individually, or because M. athalia has a narrower host range than does Vanessa cardui.
Within stands, the overall density of plants did not vary in the experiment, but the density of each plant species was low in the multispecies stands, and high in the monospecies stands. One might expect a preferred host plant to be used with high frequency when it is in a monospecific rather than a multi species stand, because a large resource patch might be more attractive than a smaller one, and may arrest foraging herbivores (Root 1973). For example Janz et al. (2005a) found that females of the butterfly Polygonia c-album spent more time and laid more eggs in patches with a high density of their preferred host. We found that the preferred plant species were oviposited upon with equal frequency whether they were in single or mixed species groups. This could mean that the mixed and monospecific stands were equally attractive, perhaps because all of the species were at least potential host plants, and that the butterflies only discriminated among plant species once they had arrived at the patch. Because M. athalia lays only one or two egg clusters a day, the high density of the host plant in the plot would not cause them to stay in the plot after oviposition, so adults are unlikely to have aggregated at the scale observed here.
Melitaea athalia have been observed to lay eggs on plants adjacent to the host plant (Wahlberg 2000; Asher et al. 2001). In this study, no eggs were found on plants abutting host plants. Further, the hosts plants used in the mixed patches corresponded to host plant preference in the single species patches, suggesting that the butterflies oviposited directly on to the chosen host plant. The precise oviposition could be an artefact of using potted plants, which distinguished them from adjacent plants, or it could be population level differences in M. athalia behaviour.
Chemical contents of the oviposition plants
The host range of M. athalia is restricted to plants producing IG secondary chemicals. Not only are IGs unlikely to be detrimental to their larvae (Harvey et al. 2005; Saastamoinen et al. 2007), but they can also be sequestered (Suomi et al. 2001; Reudler Talsma 2007), and can potentially confer protection against generalist natural enemies (Bowers 1980; Bowers and Puttick 1986; Camara 1997). Therefore we might expect plants with high IGs to be preferred. The concentrations of the two IGs aucubin and catalpol varied widely between plant species in this study (0.7–11.5% of the dry weight), and within species (0.4–12.2% in V. spicata). No correlation between oviposition preference for a host plant species and the average IG content of that species was found. This indicates that the butterflies cannot discriminate among plants based on IGs, or that they make oviposition choices based on other factors.
Other checkerspot species distinguish among suitable host species (Singer and Thomas 1987; Kuussaari et al. 2000), and among individuals of the same plant species (Singer and Lee 2000; Singer et al. 2002). At least some of this discrimination is correlated with IG content (Nieminen et al. 2003; Peñuelas et al. 2006). In natural populations Nieminen et al. (2003) found eggs of M. cinxia on plants with a higher aucubin level than neighbouring or random plants. Reudler Talsma et al. (in press) found a similar pattern in an experimental test of M. cinxia oviposition preference, and also showed that the IGs are pre-existing rather than induced by oviposition.
Melitaea athalia uses a broader range of host plants species than does M. cinxia (Wahlberg et al. 2004). Their lack of distinction among plants based on IG concentrations supports the hypothesis that increased polyphagy is associated with decreased discrimination (Bernays 1996; Janz and Nylin 1997).
Egg cluster size
Among Lepidoptera that lay eggs in clusters, such as M. athalia, variation in egg cluster size is associated with larval feeding aggregation size, as well as with vulnerability to egg desiccation, cannibalism (Clark and Faeth 1998), and rate of parasitism (Weseloh 1972). It has been found to vary geographically (e.g., Wahlberg et al. 2004), to increase with host plant density and size (Matsumato et al. 1993), and to both increase (Bergström et al. 2006) and decrease (Agnew and Singer 2000) with plant quality. We found the number of eggs in a cluster to vary greatly, but in general not in association with the host plant species. An exception was M. pratense which received significantly larger egg clusters than other species in the multispecies stands. This could indicate that the butterflies prefer this species (Bergström et al. 2006), or alternatively that the butterflies using this species have a large egg load (Agnew and Singer 2000).
While we found no clear association of IG concentration with oviposition choice, we did find a positive correlation between the number of eggs in a cluster and IG concentration within some plant species. This pattern was most clear in the transect V. spicata plants, which tended to receive larger egg clusters with increasing catalpol concentrations. There was also a non significant trend in the same direction in P. major and V. chamaedrys. Though weak, this pattern suggests that the butterflies, while not choosing plant species or plant individuals based on IGs, may have controlled the number of eggs laid in a cluster in response to IGs, or factors correlated with IG concentration. To our knowledge this association of IG concentration and egg cluster size has not been observed before.
Larval performance
Most larvae used in the performance experiment survived, even on plant species that are not known to be used by M. athalia naturally. Their performance in terms of development time, survival and weight was rather similar on all host plants except V. officinalis on which they performed poorly. The latter result corresponds with the oviposition experiment in which only three out of the 455 egg clusters were laid on V. officinalis plants. Furthermore, Schwarzwälder et al. (1997) found that in grasslands in Southern Switzerland, where V. officinalis was quite abundant, only very few caterpillars were observed feeding on it. Instead they fed on V. chamaedrys and P. lanceolata. It is interesting to note that all larvae fed M. nemorosum survived and that they developed the fastest. While other species of this genus are known host plants of M. athalia, this particular species is not a commonly listed host (though see Lepidopterologen-Arbeitsgruppe 1988; where it is reported as a host in the literature). Melampyrum nemorosum generally grows in broad-leaved forests, but also in shaded forest edges. This is a type of habitat in which adult M. athalia may occur (Lepidopterologen-Arbeitsgruppe. 1988), but it is not commonly used by M. athalia in Åland.
With the exception of these two plant species (V. officinalis and M. nemorosum), our analysis of larval performance suggests that the ovipositing butterflies would not benefit, in terms of the development of their larvae, by choosing one of the host plant species over another. One implication of this is that for larval performance it would hardly matter whether the host plant species that the females selected in our oviposition experiment occurred in a mixed-species group or in a single-species group. This is important for the large post-diapause larvae that are mobile and solitary, feeding on plants other than the one that they started on.
The larvae in this experiment were fed in Petri dishes with leaf pieces, so they did not experience whole-plant chemistry, local environmental conditions and natural enemies. It is worth noting that for the related butterfly M. cinxia, whose distribution overlaps with that of M. athalia, no systematic difference in larval performance on its two host plant species, P. lanceolata and V. spicata, were found in natural field conditions, even though in the laboratory they perform better on V. spicata than on P. lanceolata (van Nouhuys et al. 2003; Saastamoinen et al. 2007).
Overall, we found that in Åland M. athalia uses open meadows more than it does in the mainland of Finland or in Britain. The pattern of oviposition by M. athalia in our cage experiment corresponded well with the set of host species that was abundant where adults were found flying. That is, the open meadow species V. chamaedrys, V. spicata and P. lanceolata were used more than the M. pratense, which is present in the study area but grows in more sheltered habitat. The distribution of M. athalia in Åland overlaps more with the co-occurring M. cinxia than would be expected based on our knowledge of the distribution of M. athalia elsewhere. This strengthens the prediction that the two species interact indirectly through shared natural enemies (van Nouhuys and Hanski 2005).
The butterflies choose plants for oviposition in open rather than vegetated areas. Veronica chamaedrys, V. spicata and P. lanceolata received most eggs, regardless of their frequency in a patch (monospecific or part of a mixed group). This suggests that the butterfly distinguishes among plant species from within the patch, rather than at a distance.
Although the presence of IGs is an important trait distinguishing host plant species from non-host plant species for M. athalia and other Melitaeini (Wahlberg 2001), plant secondary chemistry played a smaller role in selection of suitable plants than might be expected, based on work on the congener M. cinxia (Nieminen et al. 2003; Saastamoinen et al. 2007; Reudler Talsma et al. in press). A reason could be that M. athalia occurs in more diverse habitats and has a broader host range than does M. cinxia.
While adult behaviour appears to be locally specialised to Åland, we found no association of adult habitat use with larval performance, as most plants were equally suitable for the development of the larvae. The exception was V. officinalis, which was both the least suitable for larval development, and the least preferred for oviposition, confirming a preference–performance link. Larval feeding ability was generalised, including plants from shaded habitats such as M. nemorosum one of the best plants for larval development, even though it is not used for oviposition in Åland by M. athalia. This generalisation may stem from the behaviour of the late instar M. athalia larvae, which, like some other checkerspots (Kuussaari et al. 2004; Freese et al. 2006), are solitary and can leave the oviposition plant to feed on different species in the surrounding vegetation (Wahlberg 2000). However, since oviposition comes before feeding, larval diet, regardless of its potential breadth, is restricted to the plants growing in the habitat chosen by the mother, supporting the importance of habitat and adult oviposition behaviour in defining host range.
References
Agnew K, Singer MC (2000) Does fecundity constrain the evolution of insect diet? Oikos 88:533–538
Asher J, Warren MS, Fox R, Harding P, Jeffcoate G, Jeffcoate S (2001) The millennium atlas of butterflies in Britain and Ireland. Oxford University Press, Oxford
Barron AB (2001) The life and death of Hopkins’ host-selection principle. J Insect Behav 14:725–737
Bergman KO, Askling J, Ekberg O, Ignell H, Wahlmann H, Milberg P (2004) Landscape effects on butterfly assemblages in an agricultural region. Ecography 27:619–628
Bergström A, Janz N, Nylin S (2006) Putting more eggs in the best basket: clutch-size regulation in the comma butterfly. Ecol Entomol 31:255–260
Bernays EA (1996) Selective attention and host-plant specialization. Entomol Exp Appl 80:125–131
Bowers MD (1980) Unpalatability as a defense strategy of Euphydryas phaeton (Lepidoptera: Nymphalidae). Evolution 34:586–600
Bowers MD, Puttick GM (1986) Fate of ingested iridoid glycosides in Lepidopteran herbivores. J Chem Ecol 12:169–178
Brink FA (1992) Ecologische atlas van de dagvlinders van Noordwest-Europa. Schuyt en Co. Uitgevers
Camara MD (1997) Predator responses to sequestered plant toxins in buckeye caterpillars: are tritrophic interactions locally variable? J Chem Ecol 23:2093–2106
Clark BR, Faeth SH (1998) The evolution of egg clustering in butterflies: a test of the egg desiccation hypothesis. Evol Ecol 12:543–552
Damtoft S, Franzyk H, Jensen SR (1997) Iridoid glycosides from Picconia excelsa. Phytochemistry 45:743–750
Ehrlich PR, Hanski I (2004) On the wings of checkerspots: a model system for population biology. Oxford University Press, Oxford
Franzen M, Ranius T (2004) Occurrence patterns of butterflies (Rhopalocera) in semi-natural pastures in southeastern Sweden. J Nat Conserv 12:121–135
Freese A, Benes J, Bolz R, Cizek O, Dolek M, Geyer A, Gros P, Konvicka M, Liegl A, Stettmer C (2006) Habitat use of the endangered butterfly Euphydryas maturna and forestry in Central Europe. Anim Conserv 9:388–397
Friberg M, Bergman M, Kullberg J, Wahlberg N, Wiklund C (2008) Niche separation in space and time between two sympatric sister species: a case of ecological pleiotropy. Evol Ecol 22:1–18
Gorissen D, Merckx T, Vercoutere B, Maes D (2004) Veranderd bosgebruik en dagvlinders. Waarom verdwenen dagvlinders uit bossen in Vlaanderen? Landschap 21:85–95
Hanski I, Ehrlich PR, Nieminen M, Murphy DD, Hellmann JJ, Boggs CL, McLaughlin JF (2004) Checkerspots and conservation biology. In: Ehrlich PR, Hanski I (eds) On the wings of checkerspots: a model system for population biology. Oxford University Press, New York, pp 264–287
Harvey JA, Van Nouhuys S, Biere A (2005) Effects of quantitative variation in allelochemicals in Plantago lanceolata on development of a generalist and a specialist herbivore and their endoparasitoids. J Chem Ecol 31:287–302
Jaenike J (1983) Induction of host preference in Drosophila melanogaster. Oecologia 58:320–325
Janz N (2005) The relationship between habitat selection and preference for adult and larval food resources in the polyphagous butterfly Vanessa cardui (Lepidoptera: Nymphalidae). J Insect Behav 18:767–780
Janz N, Nylin S (1997) The role of female search behaviour in determining host plant range in plant feeding insects: a test of the information processing hypothesis. Proc Roy Soc B Biol Sci 264:701–707
Janz N, Bergström A, Johansson J (2005a). Frequency dependence of host plant choice within and between patches: a large cage experiment. Evol Ecol 19:289–302
Janz N, Bergström A, Sjögren A (2005b) The role of nectar sources for oviposition decisions of the common blue butterfly Polyommatus icarus. Oikos 109:535–538
Jensen SR (1991) Plant iridoids, their biosynthesis and distribution in angiosperms. In: Harborne JB, Tomas-Barberan FA (eds) Ecological chemistry and biochemistry of plant terpenoids. Clarendon Press, Oxford, pp 133–158
Jensen SR, Nielsen BJ, Dahlgren R (1975) Iridoid compounds, their occurrence and systematic importance in the angiosperms. Botanisk Notiser 128:148–180
Kuussaari M, Singer M, Hanski I (2000) Local specialization and landscape-level influence on host use in an herbivorous insect. Ecology 81:2177–2187
Kuussaari M, van Nouhuys S, Hellmann J, Singer M (2004) Larval biology of checkerspots. In: Ehrlich PR, Hanski I (eds) On the wings of checkerspots: a model system for population biology. University Press, Oxford, pp 138–160
Lepidopterologen-Arbeitsgruppe (1988) Tagfalter und ihre Lebensräume. Schweizerischer Bund für Naturschutz, Basel
Li Y, Zou CT, Liu HW (1999) Determination of harpagide and harpagoside in Scrophularia ningpoensis by capillary electrophoresis. Chromatographia 50:358–362
Marttila O, Haahtela T, Aarnio H, Ojalainen P (1990) Suomen päiväperhoset. Kirjayhtymä, Helsinki
Matsumato K, Ito F, Tsubaki Y (1993) Egg cluster size variation in relation to the larval food abundance in Luehdorfia puziloi (Lepidoptera: Papilionidae). Res Pop Ecol 35:325–333
Mead EW, Foderaro TA, Gardner DR, Stermitz FR (1993) Iridoid glycoside sequestration by Thessalia leanira (Lepidoptera, Nymphalidae) feeding on Castilleja integra (Scrophulariaceae). J Chem Ecol 19:1155–1166
Ng D (1988) A novel level of interactions in plant-insect systems. Nature 334:611–613
Nieminen M, Suomi J, Nouhuys S, Sauri P, Riekkola ML (2003) Effect of iridoid glycoside content on oviposition host plant choice and parasitism in a specialist herbivore. J Chem Ecol 29:823–844
Papaj DR, Rausher MD (1987) Genetic differences and phenotypic plasticity as causes of variation in oviposition preference in Battus philenor. Oecologia 74:24–30
Parmesan C, Singer MC, Harris I (1995) Absence of adaptive learning from the oviposition foraging behavior of a checkerspot butterfly. Anim Behav 50:161–175
Peñuelas J, Sardans J, Stefanescu C, Parella T, Fillella I (2006) Lonicera implexa leaves bearing naturally laid eggs of the specialist herbivore Euphydryas aurinia have dramatically greater concentrations of iridoid glycosides than other leaves. J Chem Ecol 32:1925–1933
Pereyra PC, Bowers MD (1988) Iridoid glycosides as oviposition stimulants for the buckeye butterfly, Junonia coenia (Nymphalidae). J Chem Ecol 14:917–928
Pollard E (1997) A method for assessing changes in the abundance of butterflies. Biol Conserv 12:115–134
Reudler Talsma JH (2007) Cost and benefits of iridoid glycosides in multitrophic systems. Wageningen
Reudler Talsma JH, Biere A, Harvey JA, van Nouhuys S Oviposition cues for a specialist butterfly: plant chemistry and size. J Chem Ecol (in press)
Root RB (1973) Organization of a plant-arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleraceae). Ecol Monogr 43:95–124
Saastamoinen M (2007) Life-history, genotypic and environmental correlates of clutch size in the Glanville fritillary butterfly. Ecol Entomol 32:235–242
Saastamoinen M, Van Nouhuys S, Nieminen M, O’Hara B, Suomi J (2007) Development and survival of a specialist herbivore, Melitaea cinxia, on host plants producing high and low concentrations of iridoid glycosides. Ann Zool Fenn 44:70–80
Schöps K, Hanski I (2001) Population level correlation between pre-alighting and post-alighting host plant preference in the Glanville fritillary butterfly. Ecol Entomol 26:517–524
Schulman A, Heliölä J, Kuussaari M (2005) Ahvenanmaan maatalousluonnon monimuotoisuus ja maatalouden ympäristötuen vaikuttavuuden arviointi. Suomen Ympäristökeskus, Helsinki
Schwarzwälder B, Lörtscher M, Erhardt A, Zettel J (1997) Habitat utilization by the heath fritillary butterfly, Mellicta athalia spp. celadussa (Rott.)(Lepidoptera: Nymphalidae) in montane grasslands of different management. Biol Conserv 82:57–165
Selonen V (1997) Kirjoverkkoperhosen (Euphydryas maturna) ja ratamoverkkoperhosen (Mellicta athalia) populaatiorakenne ja habitaatinvalinta. Baptria 22:137–144
Shaw MR, Stefanescu C, van Nouhuys S Parasitoids of European Butterflies. In: Settele J, Shreeve TG, Konvicka M, Van Dyck H (eds) Ecology of Butterflies of Europe. Cambridge University press, Cambridge (in press)
Singer MC, Lee JR (2000) Discrimination within and between host species by a butterfly: implications for design of preference experiments. Ecol Lett 3:101–105
Singer MC, Thomas CD (1987) Variations in host preference affects movement patterns within butterfly populations. Ecology 68:1262–1267
Singer MC, Stefanescu C, Pen I (2002) When random sampling does not work: standard design falsely indicates maladaptive host preference in a butterfly. Ecol Lett 5:1–6
Stamp N (2001) Enemy-free space via host plant chemistry and dispersion: assessing the influence of tri-trophic interactions. Oecologia 128:153–163
Stermitz FR, Kader MSA, Foderaro TA Pomeroy M (1994) Iridoid glycosides from some butterflies and their larval food plants. Phytochemistry 37:997–999
Sturm S, Stuppner H (2001) Analysis of iridoid glycosides from Picrorhiza kurroa by capillary electrophoresis and high performance liquid chromatography: mass spectrometry. Chromatographia 53:612–618
Suomi J, Sirén H, Weidmer SK, Riekkola M (2001) Isolation of aucubin and catalpol from Melitaea cinxia larvae and quantification by micellar electrokinetic capillary chromatography. Anal Chim Acta 429:91–99
Suomi J, Wiedmer SK, Jussila M, Riekkola ML (2002) Analysis of eleven iridoid glycosides by micellar electrokinetic capillary chromatography (MECC) and screening of plant samples by partial filling (MECC)-electrospray ionisation mass spectrometry. J Chromatogr A 970:287–296
van Nouhuys S, Hanski I (2002) Colonization rates and distances of a host butterfly and two specific parasitoids in a fragmented landscape. J Anim Ecol 71:639–650
van Nouhuys S, Hanski I (2005) Metacommunities of butterflies, their host plants and their parasitoids. In: Holyoak M, Leibold MA, Holt RD (eds) Metacommunities: spatial dynamics and ecological communities. University of Chicago Press, Chicago, pp 99–121
van Nouhuys S, Kaartinen R (2008) A parasitoid wasp uses landmarks while monitoring potential resources. Proc Roy Soc B 275:377–385
van Nouhuys S, Singer MC, Nieminen M (2003) Spatial and temporal patterns of caterpillar performance and the suitability of two host plant species. Ecol Entomol 28:193–202
Wahlberg N (1997) Ratamoverkkoperhosen (Mellicta athalia) elinkierto Etalä-Suomessa. Baptria 22:149–153
Wahlberg N (2000) Comparative descriptions of the immature stage and ecology of five Finnish Melitaeine butterfly species (Lepidoptera: Nymphalidae). Entomol Fenn 11:67–174
Wahlberg N (2001) The phylogenetics and biochemistry of host-plant specialization in Melitaeine butterflies (Lepidoptera: Nymphalidae). Evolution 55:522–537
Wahlberg N, Klemetti T, Selonen V, Hanski I (2002) Metapopulation structure and movements in five species of checkerspot butterflies. Oecologia 130:33–43
Wahlberg N, Ehrlich PR, Boggs CL, Hanski I (2004) Bay checkerspot and Glanville fritillary compared with other species. In: Ehrlich PR Hanski I (eds) On the wings of checkerspots: a model system for population biology. Oxford University Press, Oxford, pp 219–244
Warren MS (1987a) The ecology and conservation of the heath fritillary butterfly, Mellicta athalia. III. Population dynamics and the effect of habitat management. J Appl Ecol 24:499–513
Warren MS (1987b) The ecology and conservation of the heath fritillary butterfly, Mellicta athalia. II. Adult population structure and mobility. J Appl Ecol 24:483–498
Warren MS (1987c) The ecology and conservation of the heath fritillary butterfly, Mellicta athalia. I. Host selection and phenology. J Appl Ecol 24:467–482
Warren MS, Thomas CD, Thomas JA (1984) The status of the heath fritillary Mellicta athalia Rott. in Britain. Biol Conserv 29:287–305
Weseloh RM (1972) Influence of gypsymoth egg mass dimensions and microhabitat distribution on parasitism by Ooencytrus kuwanai. Ann Entomol Soc Am 65:64–69
Acknowledgements
We thank S. Ikonen, R. Kaartinen, S. Kuokkanen, K. Lindqvist, E. Soininen and R. Wagenaar for field and laboratory assistance, and the Nåtö Biological Station for facilities in Åland. We thank A. Biere, J. A. Elzinga and I. Hanski for discussion and for helpful comments on the manuscript. This study was supported by a grant from the Earth and Life Science Foundation (ALW) of the Netherlands Organisation for Scientific Research (NWO) and the Academy of Finland Centre of Excellence Program grant number 20286. This is NIOO publication 4274.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Heikki Hokkanen.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Reudler Talsma, J.H., Torri, K. & van Nouhuys, S. Host plant use by the Heath fritillary butterfly, Melitaea athalia: plant habitat, species and chemistry. Arthropod-Plant Interactions 2, 63–75 (2008). https://doi.org/10.1007/s11829-008-9039-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-008-9039-2