Abstract
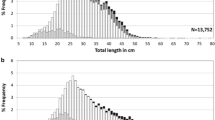
Burbot (Lota lota) are a freshwater fish that exhibits a circumpolar distribution and is considered an indicator species for climate change. Adult burbot were artificially fertilized from the Sturgeon River, Michigan and eggs were incubated until hatch. For 10 weeks following hatch larvae were photographed, along with comparable samples from the Kootenai River, Idaho. Geometric morphology was used to quantify significant morphological differences between groups (n = 510). During the yolk sac stages, body shape near yolk sacs varied, likely linked to absorption differences. In preflexion and flexion stages, variable body shape in the mid-body region coincided to head and tail positions. Mean linear morphological measurement (n = 919) showed significant differences, indicating variability of size at stage between groups. Sturgeon River preflexion larvae growth patterns were variable and exhibited allometric growth in comparison to Kootenai River larvae. This suggests that development of critical structures occurs at different rates allowing for flexibility in habitat settlement or environmental conditions. Both groups showed isometric growth patterns in the flexion stage, indicating that critical structures have developed, marking an important ontogenetic shift. Variability in larval burbot hatching, morphology, and developmental rates is important to their persistence throughout North America.











Similar content being viewed by others
References
Adams, D. C., M. L. Collyer, & A. Kaliontzopoulou, 2019. Geomorph: software for geometric morphometric analysis [available on internet at https://cran.r-project.org/package=geomorph].
Alix, M., D. Zarski, D. Chardard, P. Fontaine, & B. Schaerlinger, 2017. Deformities in newly hatched embryos of Eurasian perch populations originating from two different rearing systems. Journal of Zoology 302: 126–137.
Auer, N. A., 1982. Identification of Larval Fishes of the Great Lakes Basin with Emphasis on the Lake Michigan Drainage. Great Lakes Fisheries Commission, Ann Arbor, 776.
Berner, D., D. C. Adams, A. C. Grandchamp, & A. P. Hendry, 2008. Natural selection drives patterns of lake–stream divergence in stickleback foraging morphology. Journal of Evolutionary Biology 21: 1653–1665.
Bian, X., X. Zhang, Y. Sakurai, X. Jin, T. Gao, R. Wan, & J. Yamamoto, 2014. Temperature-mediated survival, development and hatching variation of Pacific cod Gadus macrocephalus eggs. Journal of Fish Biology 84: 85–105.
Blumstein, D. M., D. Mays, & K. T. Scribner, 2017. Spatial genetic structure and recruitment dynamics of burbot (Lota lota) in eastern Lake Michigan and Michigan tributaries. Journal of Great Lakes Research 44: 8.
Bogner, D. M., M. A. Kaemingk, & M. R. Wuellner, 2016. Consequences of hatch phenology on stages of fish recruitment. PLOS ONE 11: e0164980.
Cott, P. A., T. A. Johnston, & J. M. Gunn, 2013. Stability in life history characteristics among burbot populations across environmental gradients. Transactions of the American Fisheries Society 142: 1746–1756.
Cureton, J. C., & R. E. Broughton, 2014. Rapid morphological divergence of a stream fish in response to changes in water flow. Biology Letters 10: 20140352–20140352.
Day, T., J. Pritchard, & D. Schluter, 1994. A comparison of two sticklebacks. Evolution 48: 1723–1734.
Donner, M. T., & R. Eckmann, 2011. Diel vertical migration of larval and early-juvenile burbot optimizes survival and growth in a deep, pre-alpine lake. Freshwater Biology 56: 916–925.
Fuiman, L. A., 1983. Growth gradients in fish larvae. Journal of Fish Biology 23: 117–123.
Grabowski, T. B., V. Thorsteinsson, B. J. McAdam, & G. Marteinsdóttir, 2011. Evidence of segregated spawning in a single marine fish stock: sympatric divergence of ecotypes in Icelandic cod. PLoS ONE 6: e17528.
Haas, T. C., M. J. Blum, & D. C. Heins, 2010. Morphological responses of a stream fish to water impoundment. Biology Letters 6: 803–806.
Hardy, R., & V. L. Paragamian, 2013. A synthesis of Kootenai River burbot stock history and future management goals. Transactions of the American Fisheries Society 142: 1662–1670.
Herbing, I. H. von, 2001. Development of feeding structures in larval fish with different life histories: winter flounder and Atlantic cod. Journal of Fish Biology 59: 767–782.
Hooker, O. E., J. Barry, T. E. Van Leeuwen, A. Lyle, J. Newton, P. Cunningham, & C. E. Adams, 2016. Morphological, ecological and behavioral differentiation of sympatric profundal and pelagic Arctic charr (Salvelinus alpinus) in Loch Dughaill Scotland. Hydrobiologia 783: 209–221.
Jude, D. J., Y. Wang, S. R. Hensler, & J. Janssen, 2013. Burbot early life history strategies in the Great Lakes. Transactions of the American Fisheries Society 142: 1733–1745.
Kamler, E., 2002. Ontogeny of yolk-feeding fish: an ecological perspective. Reviews in Fish Biology and Fisheries 12: 79–103.
Kendall, A. W., E. H. Ahilstrom, & H. G. Moser, 1984. Early Life History Stages of Fishes and Their Characters Ontogeny, Systematics, Phylogeny. Allan Press, Lawrence, KS.
Koporikov, A. R., V. D. Bogdanov, L. E. Yalkovskaya, S. B. Rakitin, Yu. Ya. Khrunyk, A. S. Aldokhin, A. A. Chemagin, T. K. Tuneva, & A. V. Borodin, 2017. Ecological, morphological, and genetic diversity of burbot (Lota lota L., 1758) in large river basins of Western Siberia. Russian Journal of Ecology 48: 449–458.
Kristiansen, T., K. F. Drinkwater, R. G. Lough, & S. Sundby, 2011. Recruitment variability in North Atlantic cod and match-mismatch dynamics. PLoS ONE 6: e17456.
Kupren, K., I. Trąbska, D. Żarski, S. Krejszeff, K. Palińska-Żarska, & D. Kucharczyk, 2014. Early development and allometric growth patterns in burbot Lota lota L. Aquaculture International 22: 29–39.
Laurel, B. J., T. P. Hurst, L. A. Copeman, & M. W. Davis, 2008. The role of temperature on the growth and survival of early and late hatching Pacific cod larvae (Gadus macrocephalus). Journal of Plankton Research 30: 1051–1060.
Mansfield, P. J., D. J. Jude, D. T. Michaud, D. C. Brazo, & J. Gulvas, 1983. Distribution and abundance of larval burbot and deepwater sculpin in Lake Michigan. Transactions of the American Fisheries Society 112: 162–172.
Marcil, J., D. P. Swain, & J. A. Hutchings, 2006a. Genetic and environmental components of phenotypic variation in body shape among populations of Atlantic cod (Gadus morhua L.). Biological Journal of the Linnean Society 88: 351–365.
Marcil, J., D. P. Swain, & J. A. Hutchings, 2006b. Countergradient variation in body shape between two populations of Atlantic cod (Gadus morhua). Proceedings: Biological Sciences 273: 217–223.
Marr, J. C., 1956. The “critical period” in the early life history of marine fishes. ICES Journal of Marine Science 21: 160–170.
Marteinsdottir, G., & A. Steinarsson, 1998. Maternal influence on the size and viability of Iceland cod Gadus morhua eggs and larvae. Journal of Fish Biology 52: 1241–1258.
McIntyre, T. M., & J. A. Hutchings, 2003. Small-scale temporal and spatial variation in Atlantic cod (Gadus morhua) life history. Canadian Journal of Fisheries and Aquatic Sciences 60: 1111–1121.
McPhail, J. D., & V. L. Paragamian, 2000. Burbot biology and life history In Paragamian, Vaughn L., & Willis, David W. (eds), Burbot: Biology, Ecology and Management. Fisheries Management Section of the American Fisheries Society, Spokane, WA: 11–23,
Miler, O., & P. Fischer, 2004. Distribution and onshore migration behavior of burbot larvae in Lake Constance, Germany. Journal of Fish Biology 64: 176–185.
Miller, J. A., R. A. DiMaria, & T. P. Hurst, 2016. Patterns of larval source distribution and mixing in early life stages of Pacific cod (Gadus macrocephalus) in the southeastern Bering Sea. Deep Sea Research Part II: Topical Studies in Oceanography 134: 270–282.
Mion, J. B., R. A. Stein, & E. A. Marschall, 1998. River discharge drives survival of larval walleye. Ecological Applications 8: 17.
Neufeld, M. D., C. A. Davis, K. D. Cain, N. R. Jensen, S. C. Ireland, & C. Lewandowski, 2011. Evaluation of methods for the collection and fertilization of burbot eggs from a wild stock for conservation aquaculture operations: evaluation of methods for collection and fertilization of burbot eggs. Journal of Applied Ichthyology 27: 9–15.
Olsen, A., & M. W. Westneat, 2015. StereoMorph: an R package for the collection of 3D landmarks and curves using a stereo camera set-up [available on internet at https://aaronolsen.github.io/software/stereomorph.html].
Olsen, E. M., H. Knutsen, J. Gjøsæter, P. E. Jorde, J. A. Knutsen, & N. C. Stenseth, 2004. Life-history variation among local populations of Atlantic cod from the Norwegian Skagerrak coast. Journal of Fish Biology 64: 1725–1730.
Osse, J. W. M., J. G. M. van den Boogaart, G. M. J. van Snik, & L. van der Sluys, 1997. Priorities during early growth of fish larvae. Aquaculture 155: 249–258.
Palińska-Żarska, K., M. Woźny, M. Kamaszewski, H. Szudrowicz, P. Brzuzan, & D. Żarski, 2020. Domestication process modifies digestion ability in larvae of Eurasian perch (Perca fluviatilis), a freshwater Teleostei. Scientific Reports 10: 2211.
Pampoulie, C., T. D. Jörundsdóttir, A. Steinarsson, G. Pétursdóttir, M. Ö. Stefánsson, & A. K. Daníelsdóttir, 2006. Genetic comparison of experimental farmed strains and wild Icelandic populations of Atlantic cod (Gadus morhua L.). Aquaculture 261: 556–564.
Paragamian, V. L., R. Hardy, & B. Gunderman, 2005. Effects of regulated discharge on burbot migration. Journal of Fish Biology 66: 1199–1213.
Politis, S. N., F. T. Dahlke, I. A. E. Butts, M. A. Peck, & E. A. Trippel, 2014. Temperature, paternity and asynchronous hatching influence early developmental characteristics of larval Atlantic cod, Gadus morhua. Journal of Experimental Marine Biology and Ecology 459: 70–79.
Price, S. A., S. T. Friedman, & P. C. Wainwright, 2015. How predation shaped fish: the impact of fin spines on body form evolution across teleosts. Proceedings of the Royal Society B: Biological Sciences 282: 20151428.
R Core Team, 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [available on internet at https://www.R-project.org/].
Rasband, W. S., 1997. ImageJ. U.S. National Institutes of Health, Bethesda, Maryland [available on internet at https://imagej.nih.gov/ij/].
Recknagel, H., A. Amos, & K. R. Elmer, 2015. Morphological and ecological variation among populations and subspecies of burbot (Lota lota [L, 1758]) from the Mackenzie River Delta, Canada. The Canadian Field-Naturalist 128: 377.
Roa-Varón, A., & G. Ortí, 2009. Phylogenetic relationships among families of Gadiformes (Teleostei, Paracanthopterygii) based on nuclear and mitochondrial data. Molecular Phylogenetics and Evolution 52: 688–704.
Rohlf, F., 2015. The tps series of software. Hystrix, the Italian Journal of Mammalogy:, doi: 10.4404/hystrix-26.1-11264.
Rohlf, F. J., & D. Slice, 1990. Extensions of the procrustes method for the optimal superimposition of landmarks. Systematic Zoology 39: 40–59.
Schludermann, E., M. Tritthart, P. Humphries, & H. Keckeis, 2012. Dispersal and retention of larval fish in a potential nursery habitat of a large temperate river: an experimental study. Canadian Journal of Fisheries and Aquatic Sciences 69: 1302–1315.
Stanley, R., P. V. R. Snelgrove, B. deYoung, & R. S. Gregory, 2012. Dispersal patterns, active behavior, and flow environment during early life history of coastal cold water fishes. PLoS ONE 7: e46266.
Stapanian, M. A., W. H. Edwards, M. J. Porta, M. T. Bur, & P. M. Kocovsky, 2008. Status of burbot populations in the Laurentian Great Lakes. American Fisheries Society Symposium 59: 111–130.
Stapanian, M. A., V. L. Paragamian, C. P. Madenjian, J. R. Jackson, J. Lappalainen, M. J. Evenson, & M. D. Neufeld, 2010. Worldwide status of burbot and conservation measures. Fish and Fisheries 11: 34–56.
Synder, D., 1998. Burbot larval evidence for more than one North American species. Colorado State University, Fort Collins, Colorado.
Synder, D. E., K. R. Bestgen, & S. C. Seal, 2005. Native Cypriniform Fish Larvae of the Gila River Basin. Colorado State University, Larval Fish Laboratory, Ft. Collins, 191,
Trippel, E., G. Kraus, & F. Köster, 2005. Maternal and paternal influences on early life history traits and processes of Baltic cod Gadus morhua. Marine Ecology Progress Series 303: 259–267.
Underwood, Z. E., E. G. Mandeville, & A. W. Walters, 2016. Population connectivity and genetic structure of burbot (Lota lota) populations in the Wind River Basin, Wyoming. Hydrobiologia 765: 329–342.
Van Houdt, J., 2003. Phylogenetic relationships among Palearctic and Nearctic burbot (Lota lota): Pleistocene extinctions and recolonization. Molecular Phylogenetics and Evolution 29: 599–612.
Voesenek, C. J., F. T. Muijres, & J. L. van Leeuwen, 2018. Biomechanics of swimming in developing larval fish. The Journal of Experimental Biology 221: jeb149583.
Voss, R., H.-H. Hinrichsen, & K. Wieland, 2001. Model-supported estimation of mortality rates in Baltic cod (Gadus morhua callarias L.) larvae: the varying impact of “critical periods.” BMC Ecology 1:4.
Wang, N., & A. Appenzeller, 1998. Abundance, depth distribution, diet composition and growth of perch (Perca fluviatilis) and burbot (Lota lota) larvae and juveniles in the pelagic zone of Lake Constance. Ecology of Freshwater Fish 7: 176–183.
Wickham, H., 2016. ggplot2: Elegant Graphics for Data Analysis. Springer, New York.
Wold, P. A., K. Hoehne‐Reitan, J. Rainuzzo, & E. Kjørsvik, 2008. Allometric growth and functional development of the gut in developing cod Gadus morhua L. larvae. Journal of Fish Biology 72: 1637–1658.
Acknowledgements
We would like to thank the Kootenai Tribe of Idaho Native Fish Conservation Program and the Bonneville Power Administration Northwest Power and Conservation Council for supplying us with samples from their hatchery. Funding provided for this project was acquired through the Northern Michigan University (Development Fund, Excellence in Education, Spooner Award) and the Northern Michigan University Biology Department. Fish holding facilities, handling protocols, and experimental protocols were all approved by the Northern Michigan University Institutional Animal Care and Use Committee (IACUC # 328).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Michael Power
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ritz, T.A., Jensen, N.R. & Leonard, J.B.K. Larval morphology of North American burbot (Lota lota maculosa) from two spatially separated populations. Hydrobiologia 847, 2981–2998 (2020). https://doi.org/10.1007/s10750-020-04288-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04288-w