Abstract
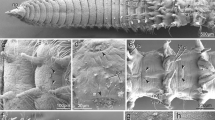
The uropygial (preen) gland is a holocrine organ unique of Aves. Although several studies have been performed on the uropygial gland of different bird species, knowledge about this gland in Columbiformes is scarce. In order to fill this gap, we analysed in detail the external morphology and the histological and histochemical features of the uropygial gland of the Eared Dove (Zenaida auriculata) in a comparative context. The uropygial gland of the Eared Dove is characterized by its pear-like shape composed of two lobes, conical and naked papilla, tubule-alveolar adenomers, a large primary storage chamber (a feature also present in other terrestrial avian species), and reticular and elastic fibres in the capsule and connective tissue surrounding the adenomers. The histochemistry showed a positive reaction to periodic acid-Schiff, Alcian Blue 2.5 and several lectins, evidencing the presence of diverse glycoconjugates in this organ. Since the uropygial gland may be independently present or absent within Columbiformes, we also used character mapping on a molecular phylogeny to infer the character states of this gland at ancestral nodes to understand its evolutionary history. The analysis shows that the presence of the uropygial gland is the ancestral state for Columbiformes and that its loss occurred more than once independently.
Zusammenfassung
Die Bürzeldrüse der Ohrflecktaube ( Zenaida auriculata ) und ihre evolutionsbiologische Geschichte innerhalb der Taubenvögel
Die Bürzeldrüse ist eine nur bei Vögeln vorkommende holokrine Drüse. Obwohl es einige Untersuchungen dieser Drüse bei unterschiedlichen Vogelarten gibt, wissen wir nicht viel über sie bei Tauben. Um diese Lücke zu schließen, analysierten wir vergleichend und im Detail die äußerlichen morphologischen sowie die histologischen und histochemischen Eigenschaften der Bürzeldrüse von Ohrflecktauben (Zenaida auriculata). Ihre Bürzeldrüse ist durch ihre Birnenform charakterisiert und setzt sich zusammen aus zwei Lappen, einer konischen und nackten Papille, tubulealveolaren Adenomen, einer großen primären Speicherkapsel (die es auch bei anderen Landvögeln gibt), retikulären und elastischen Fasern in der Kapsel und Bindegewebe um die Adenomen herum. Die Histochemie zeigte positive Reaktionen auf PAS, Alcian-Blau 2,5 und mehrere Lektine, was auf das Vorhandensein von diversen Glykokonjugaten in diesem Organ hinweist. Da die Bürzeldrüse bei Tauben unabhängig voneinander vorhanden sein oder fehlen kann, betrachteten wir ihr Vorhandensein innerhalb des molekularen Stammbaums der Tauben, um die evolutionsbiologische Geschichte der Drüse zu verstehen. Die Analyse zeigt, dass das Vorhandensein der Bürzeldrüse bei Tauben entwicklungsgeschichtlich der Normalfall war und sie mehr als einmal unabhängig voneinander verlorenging.




Similar content being viewed by others
References
Amo L, Avilés JM, Parejo D, Peña A, Rodríguez J, Tomás G (2012) Sex recognition by odour and variation in the uropygial gland secretion in starlings. J Anim Ecol 81(3):605–613
Atoji Y, Suzuky Y, Sugimura M (1989) The preputial gland of the Japanese Serow Capricornis crispus: ultrastructure and lectin-histochemistry. Acta Anat 134:245–252
Bandyopadhyay A, Bhattacharyya SP (1996) Influence of fowl uropygial gland and its secretory lipid components on the growth of skin surface bacteria of fowl. Indian J Exp Biol 34:48–52
Bandyopadhyay A, Bhattacharyya SP (1999) Influence of fowl uropygial gland and its secretory components on the growth of skin surface fungi of fowl. Indian J Exp Biol 37:48–52
Baptista LF, Trail PW, Horblit HM (1997) Family Columbidae (pigeons and doves). In: del Hoyo J, Elliot A, Sargatal J (eds) Handbook of the birds of the world, vol 4. Lynx, Barcelona, pp 60–243
Bhattacharyya SP (1972) A comparative study on the histology and histochemistry of uropygial glands. Cellule 69:113–126
Bride J, Gomot L (1978) Changes at the ecto-mesodermal interface during development of the duck preen gland. Cell Tissue Res 194:141–149
Cater DB, Lawrie NR (1950) Some histochemical and biochemical observations on the preen gland. J Physiol 3:231–243
Chiale MC, Montalti D (2013) The relationship of the feather tuft of the uropygial gland and terrestrial/aquatic birds. Rev Bras Ornitol 21:162–167
Chiale MC, Fernandez PE, Gimeno EJ, Barbeito CG, Montalti D (2014) Morphology and histology of the uropygial gland in Antarctic birds: relationship with their contact with the aquatic environment? Aust J Zool 62:367157–367165
Chiale MC, Montalti D, Flamini MA, Fernandez PE, Gimeno EJ, Barbeito CG (2015) Histological and histochemical study of the uropygial gland of Chimango Caracara (Milvago chimango, Vieillot 1816). Biotech Histochem 91:30–37
Chiale MC, Montalti D, Flamini MA, Barbeito CG (2016) The uropygial gland of the Southern Caracara (Caracara plancus; Falconidae: Falconinae): histological and histochemical aspects. Acta Zool 98(3):245–251
Czirjak GA, Pap PL, Vagasi CI, Giraudeau M, Muresan C, Mirleau P (2013) Preen gland removal increases plumage bacterial load but not that of feather degrading bacteria. Naturwissenschaften 100:145–151
Díaz AO, García AM, Goldemberg AL (2008) Glycoconjugates in the mucosa of the digestive tract of Cynoscion guatucupa: a histochemical study. Acta Histochem 110:76–85
Flamini MA, Dıaz AO, Barbeito CG, Postiansky EL (2012) Morphology, morphometry, histochemistry and lectin histochemistry of the vagina of the Plains Viscacha (Lagostomus maximus). Biotech Histochem 87:81–94
Fukui Y (1997) Epidermal growth factor inhibits morphogenesis. Dev Growth Differ 39:157–166
Gabius HJ (2000) Biological information transfer beyond the genetic code: the sugar code. Naturwissenschaften 87:108–121
Galvan I, Barbar E, Piculo R, Canto JL, Cortes V, Monros JS, Atienzar F, Proctor H (2008) Feather mites and birds: an interaction mediated by uropygial gland size? J Evol Biol 21:133–144
Gibbs D, Barnes E, Cox J (2010) A guide to the pigeons and doves of the world. Helm, London, p 615
Goldstein IJ, Hayes CE (1978) The lecyins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem 35:127–340
Goldstein IJ, Hughes RC, Monsigny M, Osawa T, Sharon N (1980) What should be called a lectin? Nature 285:66
Harem IS, Kocak-Harem M, Turan-Kozlu T, Akaydin- Bozkurt Y, Karadag-Sari E, Altunay H (2010) Histologic structure of the gland of the Osprey (Pandion haliaetus). J Zoo Wildl Med 41:148–151
Heupink TH, van Grouw H, Lambert DM (2014) The mysterious Spotted Green Pigeon and its relation to the Dodo and its kindred. Evol Biol 14:136
Hirao A, Aoyama M, Sugita S (2009) The role of uropygial gland on sexual behaviour in Domestic Chicken Gallus gallus domesticus. Behav Process 80:115–120
Hou HC (1928) Studies on the glandula uropygialis of birds. Am J Physiol 85:380
Iwamoto S, Nakayama H, Doi K (1998) Morphological and morphometrical study on the dorsal skin of Wistar and WBN/ILAHt rats in their development age. Evaluation of the proliferation and apoptotic processes. Histol Histopathol 13:981–988
Jacob J (1976) Bird waxes. In: Kolattukudy PE (ed) Chemistry and biochemistry of natural waxes. Elsevier, Amsterdam, pp 93–146
Jacob J, Ziswiler V (1982) The uropygial gland. In: Farner DS, King JR, Parkes KC (eds) Avian biology, vol 6. Academic Press, New York, pp 199–324
Johnston DW (1988) A morphological atlas of the avian uropygial gland. Bull Br Mus (Nat Hist), Zool Ser 54(5):199–259
Kamiya S, Izumisawa Y, Tsukushi M, Amasaki H, Daigo M (1986) Histochemical studies on polysaccharides in the uropygial gland of ducks. Bull Nippon Vet Zootech Coll 35:1–7
Kolattukudy PE (1981) Avian uropygial (preen) gland. Methods Enzimol 72:714–718
Kozlu T, Akaydin-Bozkurt Y, Ates S (2011) A macroanatomical and histological study of the uropygial gland in the White Stork (Ciconia ciconia). Int J Morphol 29:723–726
Lapiedra O, Sol D, Carranza S, Beaulieu JM (2013) Behavioural changes and the adaptive diversification of pigeons and doves. Proc R Soc B 280:2012–2893
Lucas AM, Stettenheim PR (1972) Uropygial gland. In: Lucas AM, Stettenheim PR (eds) Avian anatomy. Part II. Agricultural handbook. Department of Agriculture, U.S. Government Printing Office, Washington, pp 613–626
Maddison WP, Maddison DR (2018) Mesquite: a modular system for evolutionary analysis. Version 3.5. http://www.mesquiteproject.org. Accessed 30 June 2018
Meyer W, Seegers U, Schnaper A, Neuhaus H, Himstedt W, Toepfer-Petersen E (2007) Possible antimicrobial defense by free sugars on the epidermal surface of aquatic vertebrates. Aquat Biol 1:167–175
Møller AP, Czirjak GA, Heeb P (2009) Feather microorganisms and uropygial antimicrobial defenses in a colonial passerine bird. Funct Ecol 23:1097–1102
Montalti D, Salibian A (2000) Uropygial gland size and avian habitat. Ornitol Neotrop 11:297–306
Montalti D, Gutierrez AM, Salibian A (1998) Tecnica quirúrgica para la ablación de la glándula uropigia en la Paloma Casera Columba livia. Rev Bras Biol 58:193–196
Montalti D, Quiroga MA, Massone AR, Idiart JR, Salibian A (2001) Histochemical and lectin-histochemical studies of the secretion from the uropygial gland of the Rock Dove Columba livia (Columbidae-Columbiformes). Braz J Morphol Sci 18:35–39
Montalti D, Gutierrez AM, Reboredo GR, Salibián A (2005) The chemical composition of the uropygial gland secretion of Rock Dove. Comp Biochem Physiol A 140:275–279
Moreno-Rueda G (2017) Preen oil and bird fitness: a critical review of the evidence. Biol Rev Camb Philos Soc 92:2131–2143
Niemann C, Horsley V (2012) Development and homeostasis of the sebaceous gland. Semin Cell Dev Biol 8:928–936
Ookusa Y, Takana K, Nagashima M, Hirano H (1983) Distribution of glycoconjugates in normal human skin using biotinyl lectins and avidin-horseradish peroxidase. Histochemistry 79:1–7
Paris P (1912) Sur la présence des corpuscules de Herbst dans la glande uropygienne des Oiseaux. Compte Rendus Acad Sci Paris 155:786–787
Rehorek SJ, Wu JL, Smith TD, Beeching SC (2017) Embryogenesis of the uropygial glands in the Laysan Albatross (Phoebastria immutabilis (Rothschild, 1893): Procellariiformes). Anat Rec 300:1420–1428
Salibian A, Montalti D (2009) Physiological and biochemical aspects of the avian uropygial gland. Braz J Biol 69:437–446
Sawad AA (2006) Morphological and histological study of uropygial gland in the Moorhen (G. gallinula C. chloropus). Int J Poult Sci 50:938–940
Shawkey MD, Pillai SR, Hill GE (2003) Chemical warfare? Effects of uropygial oil in feather degrading bacteria. J Avian Biol 34(4):345–349
Soler JJ, Peralta-Sánchez JM, Martin-Platero AM, Martin-Vivaldi M, Martínez-Bueno M, Møller AP (2012) The evolution of size of the uropygial gland: mutualistic feather mites and uropygial secretion reduce bacterial loads of eggshells and hatching failures of European birds. J Evol Biol 25:1179–1191
Springer MS, Gatesy J (2018) Evolution of the MC5R gene in placental mammals with evidence for its inactivation in multiple lineages that lack sebaceous glands. Mol Phylogenetics Evol 120:364–374
Suvarna SK, Layton C, Bancroft JD (2012) Bancroft’s theory and practice of histological techniques, 7th edn. Elsevier, Amsterdam, p 604
Thomas AL, Maekawa F, Kawashima T, Sakamoto H, Sakamoto T, Davis P, Dores RM (2018) Analyzing the effects of co-expression of chick (Gallus gallus) melanocortin receptors with either chick MRAP1 or MRAP2 in CHO cells on sensitivity to ACTH (1-24) or ACTH (1-13)NH2: implications for the avian HPA axis and avian melanocortin circuits in the hypothalamus. Gen Comp Endocrinol 256:50–56
Yashpal M, Kumari U, Mittal S, Mittal AK (2014) Glycoproteins in the buccal epithelium of a carp, Cirrhinus mrigala (Pisces, Cyprinidae): a histochemical profile. Anat Histol Embryol 43:116–132
Yasui T, Tsukise A, Fukui K, Kuwahara Y, Meyer W (2005) Aspects of glycoconjugates production and lysozyme and defensins expression of the ceruminous glands of the horse (Equus przewalskii f. dom.). Eur J Morphol 42(3):127–134
Zanuzzi CN, Barbeito CG, Ortíz ML, Lozza FA, Fontana PA, Portiansky EL, Gimeno EJ (2010) Glycoconjugate histochemistry in the small and large intestine of normal and Solanum glaucophyllum-intoxicated rabbits. Res Vet Sci 89:214–222
Zhang JX, Wei W, Zhang JH, Yang WG (2010) Uropygial gland secreted alkanols contribute to olfactory sex signals in Budgerigars. Chem Senses 35(1):375–382
Acknowledgements
We appreciate the improvements made to the English language of this article by Peter Lowther through the Association of Field Ornithologists’ programme of editorial assistance. We are grateful to CONICET for permanent support. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Fusani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chiale, M.C., Carril, J., Montalti, D. et al. The uropygial gland of the Eared Dove and its evolutionary history within the Columbiformes (Aves). J Ornithol 160, 1171–1181 (2019). https://doi.org/10.1007/s10336-019-01691-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-019-01691-6