Abstract
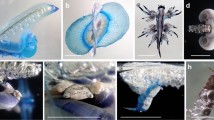
The life cycle of Lychnorhiza lucerna (Scyphozoa: Rhizostomeae) and the settlement preferences of its larvae were studied using laboratory-based rearing experiments. Mature medusae of L. lucerna were collected from the beach of the Río de la Plata estuary, Argentina. This species displayed the typical metagenetic, (i.e. medusoid and polypoid), life cycle reported for other rhizostomes. The fertilized eggs developed into motile and short lived planulae. The majority of planulae settled on the air-water interface (p < 0.001). Of those that settled on the settlement plates provided, no significant differences were observed between styrene slides, glass slides and shells of the bivalve Mactra isabelleana (p > 0.05). No planulae settled on stones. Several hours after planulae settled, they metamorphosed into sessile four-tentacled scyphistomae. Most scyphistomae attached onto the air-water interface. At 19–22°C, the scyphistomae grew up to 22 tentacles and reached 1,500 μm height. The scyphistomae increased their numbers by means of formation of podocysts from which new polyps emerged and strobilated. Strobilation occurred 46 days after settlement. Only polydisk strobilation was observed and each strobila always produced three ephyrae. After releasing ephyrae, strobilae returned to normal scyphistomae and were capable of repeating strobilation. A single founder polyp was estimated to produce up to 60 ephyrae over 4 months. Ephyrae developed into metephyrae 15 days after release at 19–22°C. In this paper we describe the morphological and some behavioural features of L. lucerna in the polypoid and early medusoid stages.







Similar content being viewed by others
References
Álvarez Colombo G, Mianzan HW, Madirolas A (2003) Acoustic characterization of gelatinous-plankton aggregations: four case studies from the Argentinean continental shelf. ICES J Mar Sci 60:650–657. doi:https://doi.org/10.1016/S1054-3139(03)00051-1
Arai MN (1997) A functional biology of Scyphozoa. Chapman & Hall, London
Berrill N (1949) Developmental analysis of Scyphomedusae. Biol Rev Camb Philos Soc 24:393–410. doi:https://doi.org/10.1111/j.1469-185X.1949.tb00581.x
Bigelow RP (1900) The anatomy and development of Cassiopea xamachana. Mem Boston Soc Nat Hist 5:191–236
Blanquet RS (1972) Structural and chemical aspects of the podocyst cuticle of the scyphozoan medusa, Chrysaora quinquecirrha. Biol Bull 142:1–10. doi:https://doi.org/10.2307/1540241
Brewer RH, Feingold JS (1991) The effect of temperature on the benthic stages of Cyanea (Cnidaria: Scyphozoa), and their seasonal distribution in the Niantic River estuary, Connecticut. J Exp Mar Biol Ecol 152:49–60. doi:https://doi.org/10.1016/0022-0981(91)90134-I
Brodeur RD, Mills CE, Overland JE, Walters GE, Schumacher JD (1999) Evidence for a substantial increase in gelatinous zooplankton in the Bering Sea, with possible links to climate change. Fish Oceanogr 8:296–306. doi:https://doi.org/10.1046/j.1365-2419.1999.00115.x
Calder DR (1973) Laboratory observations on the life history of Rhopilema verrilli (Scyphozoa: Rhizostomeae). Mar Biol (Berl) 21:109–114. doi:https://doi.org/10.1007/BF00354606
Calder DR (1982) Life history of the Cannonball jellyfish, Stomolophus meleagris L. Agassiz, 1860 (Scyphozoa, Rhizostomida). Biol Bull 162:149–162. doi:https://doi.org/10.2307/1540810
Cargo DG (1971) The sessile stages of a Scyphozoan identified as Rhopilema verrilli. Tulane Stud Zool Bot 17:31–34
Cargo DG, Rabenold GE (1980) Observations on the asexual reproductive activities of the sessile stages of the sea nettle Chrysaora quinquecirrha (Scyphozoa). Estuaries 3:20–27. doi:https://doi.org/10.2307/1351931
Cargo DG, Schultz LP (1966) Notes on the biology of the Sea Nettle, Chrysaora quinquecirrha, in Chesapeake Bay. Chesap Sci 7:95–100. doi:https://doi.org/10.2307/1351129
Chen J, Ding G (1983) Effect of temperature on strobilation of jellyfish (Rhopilema esculenta Kishinouye—Scyphozoa, Rhizostomeae). Acta Zoologica Sin 29:195–206
Chen J, Ding G (1984) Effect of light on the strobilation of edible medusa, Rhopilema esculenta Kishinouye (Cnidaria, Scyphozoa). Oceanologia Limnol sin 15:310–316
Claus C (1890) Über die Entwicklung des Scyphostoma von Cotylorhiza, Aurelia und Chrysaora, sowie ueber die systematische Stellung der Scyphomedusen. I. Arbeiten Zoologischen Inst Univ Wien Zoologischen Stn Triest 9:85–128
Claus C (1893) Über die Entwicklung des Scyphostoma von Cotylorhiza, Aurelia, und Chrysaora, sowie ueber die systematische Stellung der Scyphomedusen. II. Arbeiten Zoologischen Inst Univ Wien Zoologischen Stn Triest 10:1–70
Ding G, Chen J (1981) The life history of Rhopilema esculenta Kishinouye. J Fish China 5:93–104 Suichan Xuebao
Gohar HAF, Eisawy AM (1960) The development of Cassiopea andromeda (Scyphomedusae). Publ Mar Biol Sta Ghardaqa Red Sea 11:147–190
Graham WM, Pagès F, Hammer WM (2001) A physical context for gelatinous zooplankton aggregations: a review. Hydrobiologia 451:199–212. doi:https://doi.org/10.1023/A:1011876004427
Guo P (1990) Effect of nutritional condition on the formation and germination of the podocyst of scyphistomae of Rhopilema esculenta Kishinouye. J Fish China 14:206–211 Suichan Xuebao
Haeckel E (1880) Das System der Medusen. I, 2: system der Acraspeden. Gustav Fischer, Jena, pp 361–672
Hofmann DK, Honegger TG (1990) Bud formation and metamorphosis in Cassiopea andromeda (Cnidaria: Scyphozoa): a developmental and ultraestructural study. Mar Biol (Berl) 105:509–518. doi:https://doi.org/10.1007/BF01316322
Holst S, Sötje I, Tiemann H, Jarms G (2007) Life cycle of the rhizostome jellyfish Rhizostoma octopus (L.) (Scyphozoa, Rhizostomeae), with studies on cnidocysts and statoliths. Mar Biol (Berl) 151:1695–1710. doi:https://doi.org/10.1007/s00227-006-0594-8
Jarms G (2007) The polyps of the Scyphozoa and their impact on jellyfish blooms. Second International Jellyfish Blooms, 24–27 June, Gold Coast, Australia
Kawahara M, Uye S-i, Ohtsu K, Iizumi H (2006) Unusual population explosion of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) in East Asian waters. Mar Ecol Prog Ser 307:161–173. doi:https://doi.org/10.3354/meps307161
Keen SL (1987) Recruitment of Aurelia aurita (Cnidaria: Scyophozoa) larvae is position-dependent, and independent of conespecific density, within a settling surface. Mar Ecol Prog Ser 38:151–160. doi:https://doi.org/10.3354/meps038151
Kikinger R (1992) Cotylorhiza tuberculata (Cnidaria: Scyphozoa) - Life history of a stationary population. Mar Ecol (Berl) 13:333–362. doi:https://doi.org/10.1111/j.1439-0485.1992.tb00359.x
Kramp PL (1961) Synopsis of the medusae of the world. J Mar Biol Assoc UK 40:7–469
Kramp PL (1970) Zoogeographical studies on Rhizostomeae (Scyphozoa). Vidensk Meddr dansk naturh Foren 133:7–30
Lange J, Kaiser R (1995) The mantenance of pelagic jellyfish in the Zoo-Aquarium Berlin. Int Zoo Yb 34:59–64. doi:https://doi.org/10.1111/j.1748-1090.1995.tb00658.x
Loeb MJ (1973) The effect of light on strobilation in the Chesapeake Bay sea nettle Chrysaora quinquecirrha. Mar Biol (Berlin) 20:144–147. doi:https://doi.org/10.1007/BF00351452
Loeb MJ (1974) Strobilation in the Chesapeake Bay sea nettle Chrysaora quinquecirrha II. Partial characterization of the neck-inducing factor from strobilating polyps. Comp Biochem Physiol 47:291–301. doi:https://doi.org/10.1016/0300-9629(74)90073-5
Lotan A, Ben-Hillel R, Loya Y (1992) Life cycle of Rhopilema nomadica: a new immigrant scyphomedusan in the Mediterranean. Mar Biol (Berl) 112:237–242. doi:https://doi.org/10.1007/BF00702467
Ludwig FD (1969) Die Zooxanthellen bei Cassiopea andromeda Eschscholtz 1829 (Polyp-Stadium) und ihre Bedeutung für die Strobilation. Zool Jb 86:238–277 Abt Anat Ontog Tiere
Magnusen JE (1980) Epidermal cell movement during podocyst formation in Chrysaora quinquecirrha. In: Tardent O, Tardent R (eds) Developmental and cellular biology of coelenterates. Elsevier/North Holland Biomedical Press, Amsterdam, pp 435–440
Mianzan HW (1986) Estudio sistemático y bioecológico de algunas medusas Scyphozoa de la región subantártica. PhD, La Plata
Mianzan HW, Cornelius PFS (1999) Cubomedusae and Scyphomedusae. In: Boltovskoy D (ed) South Atlantic Zooplankton. Blackuys Publishers, pp 513–559
Mills CE (2001) Jellyfish Blooms: are populations increasing globally in response to changing ocean conditions? Hydrobiologia 451:55–68. doi:https://doi.org/10.1023/A:1011888006302
Morandini AC (2003) Estrutura populacional de Chrysaora lactea e Lychnorhiza lucerna (Cnidaria, Scyphozoa) em amostras de plâncton, com a redescrição das espécies. PhD. Instituto de Biociências da Universidade de São Paulo, São Paulo
Morandini AC, Lang da Silveira F, Jarms G (2004) The life cycle of Chrysaora lactea Eschscholtz, 1829 (Cnidaria, Scyphozoa) with notes on the scyphistoma stage of three other species. Hydrobiologia 530/531:347–354. doi:https://doi.org/10.1007/s10750-004-2694-0
Müller MI, Cadaveira ML, Masakazu O (2003) Cultivo de rotíferos en Argentina - Primer alimento para larvas de peces marinos VIII Jornadas Nacionales de Ciencias del Mar. Mar del Plata, Argentina
Neumann R (1977) Polyp morphogenesis in a scyphozoan: evidence for a head inhibitor from the presumptive foot end in vegetative buds of Cassiopea andromeda. Wilhelm Roux′s Archs devl Biol 183:79–83
Nogueira M Jr, Haddad MA (2005) Lychnorhiza lucerna Haeckel (Scyphozoa, Rhizostomeae) and Libinia ferreirae Brito Capello (Decapoda, Majidae) association in southern Brazil. Rev Bras Zoologia 22:908–912
Olmon JE, Webb KL (1974) Metabolism of 131I in relation to strobilation of Aurelia aurita L. (Scyphozoa). J Exp Mar Biol Ecol 16:113–122. doi:https://doi.org/10.1016/0022-0981(74)90014-8
Paspaleff BW (1938) Über die Entwicklung von Rhizostoma pulmo Agass. Trud chernomorsk biol Sta Varna 7:1–25
Pitt KA (2000) Life history and settlement preferences of the edible jellyfish Catostylus mosaicus (Scyphozoa: Rhizostomeae). Mar Biol (Berl) 136:269–279. doi:https://doi.org/10.1007/s002270050685
Rahat M, Adar O (1980) Effect of symbiotic zooxanthellae and temperature on budding and strobilation in Cassiopeia andromeda (Eschscholz). Biol Bull 159:394–401. doi:https://doi.org/10.2307/1541102
Raskoff KA, Sommer FA, Hamner WM, Cross KM (2003) Collection and culture techniques for gelatinous zooplancton. Biol Bull 204:68–80. doi:https://doi.org/10.2307/1543497
Rippingale RJ, Kelly SJ (1995) Reproduction and survival of Phyllorhiza punctata (Cnidaria: Rhizostomeae) in a seasonally fluctuating regime in western Australia. Mar Freshw Res 46:1145–1151. doi:https://doi.org/10.1071/MF9951145
Rottini Sandrini L, Avian M (1983) Biological cycle of Pelagia noctiluca: morphological aspects of the development from planula to ephyra. Mar Biol (Berl) 74:169–174. doi:https://doi.org/10.1007/BF00413920
Russell FRS (1970) II Pelagic Scyphozoa with a supplement to the first volume on hydromedusae The medusae of the British isles. Cambridge University Press, New York
Sokal RR, Rohlf FJ (1999) Introducción a la Bioestadística. Editorial Reverté, Barcelona
Spangenberg DB (1965) Cultivation of the life stages of Aurelia aurita under controlled conditions. J Exp Zool 159:303–318. doi:https://doi.org/10.1002/jez.1401590303
Spangenberg DB (1971) Thyroxine induced metamorphosis in Aurelia. J Exp Zool 178:183–194. doi:https://doi.org/10.1002/jez.1401780205
Sugiura Y (1963) On the life history of rhizostome medusa I. Mastigias papua L. Agassiz. Annot Zool Jpn 36:194–202
Sugiura Y (1966) On the life-history of Rhizostome medusae IV. Cephea cephea. Embryologia (Nagoya) 9:105–122
Tronolone VB, Morandini AC, Migotto AE (2002) On the occurrence of scyphozoan ephyrae (Cnidaria, Scyphozoa, Semaeostomeae and Rhizostomeae) in the Southeastern Brazilian coast. Biota Neotropica 2:1–18
Uchida T (1926) The anatomy and development of a rhizostome medusa, Mastigias papua L. Agassiz, with observations on the phylogeny of Rhizostomeae. J Fac Sci imp Univ Tokyo (IV: Zool) 1:45–95
Urien CM (1972) Río de la Plata estuarine environment. Geol Soc Am Memoir 133:213–233
Vannucci M (1951) Hydrozoa e Scyphozoa existentes no Instituto Paulista de Oceanografia I. Bol Inst Paul Oceanografia 2:69–104
Vannucci-Mendes M (1944) Sôbre a larva de Dibothriorhynchus dinoi, sp. n. parasita dos Rhizostomata. (Cestoda, Tetrarhynchidea). Arq Museu Paranaense 4:47–82
Zamponi MO (2002) The association between medusa Lychnorhiza lucerna (Scyphomedusae, Rhizostomeae) and Decapod Libinia spinosa (Brachyura, Majidae) recorded for the first time in neritic waters of Argentina. Russ J Mar Biol 28:267–269. doi:https://doi.org/10.1023/A:1020229328660
Acknowledgments
We thank Mundo Marino, Mundo Marino Foundation and Miguel Marchi for providing support and facilities in jellyfish collection. We also thank Dr. Jack Costello and two anonymous reviewers for improving this manuscript and Dr. M. D. Viñas for allowing us the use of temperature-controlled chamber. Finally we are grateful to the Aquaculture Project (INIDEP) and especially to Federico Bianca who provided us sea water, rotifers and Artemia eggs. This work was supported by Fundación Antorchas 13900–13, CONICET PIP 5009, FONCyT PICT 1553, the Inter-American Institute for Global Change Research CRN-2076, which is supported by the US National Science Foundation (Grant GEO-0452325), and Japan Science Promotion Society (No 16405001). This is INIDEP contribution No 1488.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S.A. Poulet.
Rights and permissions
About this article
Cite this article
Schiariti, A., Kawahara, M., Uye, S. et al. Life cycle of the jellyfish Lychnorhiza lucerna (Scyphozoa: Rhizostomeae). Mar Biol 156, 1–12 (2008). https://doi.org/10.1007/s00227-008-1050-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-008-1050-8