calsfoundation@cals.org
Bryozoans
aka: Ectoprocta
aka: Moss Animals
Bryozoans (commonly called moss animals) are generally sessile, colonial invertebrates that belong to the phylum Bryozoa (or Ectoprocta), which is sometimes combined with two other phyla (Phoronida and Brachiopoda) to form a possible clade within the Deuterostomia. The three are sometimes referred to as the Lophophorata. Fossil bryozoans commonly found in Arkansas can be divided into two broad groups: the lacy colonies and the twig-shaped colonies. One fossil, the Archimedes, is especially abundant in northwestern Arkansas. In the southeastern United States, large gelatinous colonies of P. magnifica are a common sight, sometimes called “dinosaur snot.” These are often seen at Lake Wilhelmina in Polk County, and some have been reported in eastern Arkansas County.
The primary uniting characteristic of this clade is a unique feeding structure called a lophophore, which is a hollow ciliated tentacular feeding structure. However, there is a three-fold disagreement over both morphological and molecular phylogenetic analyses as follows: (1) relationships of bryozoans with entoprocts (“anus inside”), (2) whether bryozoans should be grouped together with phoronids (horseshoe worms) and brachiopods in the Lophophorata, and (3) whether bryozoans should be considered protostomes or deuterostomes, the two major groups that account for all moderately complex animals. Researchers are also at odds about what is the phylum’s closest relative, but a molecular investigation using mitochondrial DNA sequences suggests that the Bryozoa may be related to the Chaetognatha (arrow worms). However, as a group, lophophorates show no clear affinity with any other invertebrates. It is also unclear whether the phylum Bryozoa is a monophyletic group (i.e., whether they include all and only a single ancestor species and all its descendants) because bryozoan evolutionary relationships to other phyla remain inexact.
Bryozoans are primarily a marine group ranging from 4,000 to 4,500 recognized species. One genus (Monobryozoon) is solitary, and the remainder are colonial species. Most marine species inhabit tropical waters less than 100 m (330 ft.) deep, but a few occur in oceanic trenches especially around cold seeps, while others are found in polar waters. In addition, a few members of a mostly marine class prefer brackish water. Some in the order Ctenostomata are exclusively freshwater inhabitants, while others prefer brackish water but can still survive in fresh water. Most bryozoans occur in both still and slightly running alkaline waters at temperatures < 30°C (86°F) and flourish at 15 to 28°C (59 to 82°F).
There are only 100 or more species known from fresh water and about twenty-four to twenty-nine recognized species of freshwater bryozoans in the Nearctic realm. In addition, all known species from North America have been reported east of the Mississippi River and north of the 39th parallel. A total of nineteen of the twenty-four bryozoan species occur in states bordering the Great Lakes, and an additional two species are described only from New England. Relatively little is known about freshwater bryozoans west of Ontario, Canada, and the Mississippi River. In fact, most states and provinces have no published records of bryozoans.
Freshwater bryozoans are unequally classified among two major groups: (1) the exclusively freshwater class Phylactolaemata (about seventy species and seven families); and (2) the mostly marine order Cheilostomata containing about fifty families within the vastly larger class Gymnolaemata, the tubular bryozoans. In the Nearctic region, phylactolaemates are said to be represented by at least thirty-two species and the ctenostomes by four species. The Phylactolaemata include the genera Lophopodella, Pectinatella, and Plumatella. The Gymnolaemata contains those that are chitinous or calcified cystid colonies. Two subclasses of these groups are the Eurystomata and Stenolaemata.
Bryozoans have a rich fossil record (about 15,000 bryozoan species are known) that extends back to the Early Ordovician, making them the last major phylum to appear in the fossil record. Late Mesozoic and Cenozoic bryozoans are found in deposits located in the Caribbean, especially Costa Rica, the Dominican Republic, Panama, and Venezuela, and the Gulf Coast region of the United States, particularly Alabama, Florida, and Mississippi. By the Arenigian stage (the time interval during the Lower Ordovician period and also to the suite of rocks that were deposited during this interval) of the Early Ordovician period (about 480 million years ago), all the modern orders of stenolaemates were present, and the ctenostome order of gymnolaemates had appeared by the Middle Ordovician (about 465 million years ago). Fossils of cheilostomates, another order of gymnolaemates, first appear in the mid-Jurassic (about 172 million years ago), and these have been the most abundant and diverse bryozoans from the Cretaceous to the present. Fossils of the soft, freshwater phylactolaemates are very rare and appear in and after the Late Permian (about 260 million years ago) and consist entirely of their durable statoblasts (encapsulated products of asexual reproduction). There are no known fossils of freshwater members of other classes.
In Arkansas, fossil bryozoans are commonly found and can be divided into the lacy colonies and the twig-shaped colonies. Probably the most famous bryozoan fossil is Archimedes. It consists of a central spiral-shaped axis fringed by a lacy colony. This screw-like fossil is especially abundant in northwestern Arkansas in the Pitkin Limestone deposits. Some rahbdomesid and fenestellid bryozoan fossils have been found in southern Columbia County. Other well-known fossil bryozoans found in the state are those in the genera Fenestrella, Pentremites, Prismopora, Rhombopora and Septopora.
Many species of bryozoans are common and easily identified, whereas others are distinguished only by diminutive and sometimes uncertain morphological features. The soft tissues of bryozoans offer few reliable structures for species identification, and even molecular genetics tools may not be sufficiently refined to detect cryptic species. Hard parts, when they are obtainable, tend to be much more uniform and demonstrable. Traditional taxonomists have been obligated to rely on such variable features as layout of the gut and counts of tentacles.
Many difficult taxonomic issues occur within the freshwater family Plumatellidae, which contains about eight genera and comprises about half of the Nearctic species. By contrast, phylactolaemate bryozoans have taxonomically useful hard parts in their encapsulated dormant buds or statoblasts. These provide a treasure of morphological characters, such as relative shapes and dimensions, as well as tiny tubercles and net-like lines that are believed to be species-specific. In most cases, the statoblast alone is sufficient to identify a bryozoan species. All statoblasts are formed like two halves of a clam shell, which split apart at the time of germination. Each half, or valve, is normally composed of two layers: an inner capsule and an outer periblast. The capsule has no taxonomic value, but the periblast bears a wide variety of morphological features. In floatoblasts (free statoblasts), the periblast includes the ring of gas-filled chambers and in the center of this ring is an area called the fenestra. By examining the relative dimensions of the periblast and its fenestra, those two features can be useful as taxonomic characters. In fact, when statoblasts are absent, many phylactolaemates cannot be positively identified at the specific level. The search for ever more elusive taxonomic features has predictably led to important features being detected only through scanning electron microscopy (SEM). More recently, the application of non-destructive imaging methods for quantitative taxonomy of bryozoans have been successful using microcomputed tomography (micro-CT).
Morphologically, bryozoan colonies are composed of numerous physically and physiologically connected zooids that are not fully independent individuals. The feeding organ is a cluster of ciliated tentacles called a lophophore, which captures particles suspended in the water. The outermost part of a zooid is a non-living layer called an ectocyst, composed of either chitin or a slick mucopolysaccharide. The inner part of the zooid is the polypide, which includes the lophophore and the entire digestive tract. When the zooid is alarmed, the entire polypide is quickly retracted and the lophophore becomes fully protected. Statoblasts are essentially tiny packages of yolky material and germinal cells enclosed within a bilayered case. There are several types of statoblasts: floatoblasts, sessoblasts, and piptoblasts. The floatoblasts are freely released and have a peripheral ring of gas-filled chambers for buoyancy, and sessoblasts are firmly attached to the substrate. The piptoblast is composed of the capsule only without a periblast.
All bryozoans are colonial except one genus, Monobryozon. Thus, bryozoans typically form sessile colonies that live in marine and freshwater environments, and consist of individual zooids that are about 0.5 mm (0.02 in.) long. While zooids are microscopic, bryozoan colonies range in size from one cm (0.39 in.) to over one meter (3.3 ft.) across. However, most colonies are under 10 cm (3.9 in.) across. Zooids are not fully independent organisms. Colonies possess feeding zooids known as autozooids, and some groups contain non-feeding specialist zooids called heterozooids such as those that serve as hatcheries for fertilized eggs, while some classes have specialized zooids used for defense. Colonial members are genetically identical and work together like the organs of a larger organism.
Marine bryozoan colonies tend to grow on or attach to solid substrates such as shells, rocks, large brown algae, mangrove roots, ship bottoms, and the undersides of icebergs. They live at all water depths and all latitudes. Growth forms of bryozoans are sometimes encrusted and coral-like and create miniature “reefs” in shallow water habitats. Others are more dense and bush-like colonies or gelatinous spaghetti-like masses. Encrusting forms can have mineralized exoskeletons and can grow on the shells or the exoskeletons of other invertebrates and look very much like small corals.
The bryozoan body plan is complicated. The cystid comprises the outer body wall, and the exoskeleton may be organic or made up of calcium carbonate (CaCO3). The polypide includes the internal organs, muscles, nervous system, and tentacle crown (the lophophore). The opening in the cystid through which the tentacle crown extends is termed the orifice. It has an operculum, which is a flaplike chintinous covering in the cheilostomes.
Bryozoans undergo radial, holoblastic, almost equal cleavage to form a coeloblastula (a blastula with a fluid-filled cavity) or a stereoblastula (a blastula without a clear central cavity). Ensuing development differs greatly among groups, but in all cases it involves a dispersal free-swimming form. Because bryozoans display no discrete cephalization and live a sessile lifestyle, their nervous organs are primitive. They do possess a cerebral ganglion (neuronal mass) that serves as a brain that lies on the oral side of the pharynx. A circumoral nerve ring arises from this structure, with nerves extending from the ring and ganglion to the viscera into each tentacle. Bryozoans have no specialized sense organs, but ciliated tentacles act as sensors.
In terms of respiration and circulation in the Bryozoa, there are no distinct respiratory organs, heart, or blood vessels. Rather, zooids use various methods to share nutrients and absorb oxygen and eliminate carbon dioxide via simple diffusion. Some bryozoan groups have very large openings in their body walls, allowing coelomic fluid to circulate freely. In other groups, the internal funiculi of adjacent zooids connect by means of small pores in the body wall.
The digestive system in some species includes a proximal stomach that forms a muscular gizzard lined with chitinous teeth that crush armored prey items that possess opaline silica such as diatoms. Wave-like peristaltic contractions move the food through their stomach for digestion. The distal section of the stomach is lined with cilia that compress undigested solids, which then pass through the intestine and out through the anus.
The excretory system of bryozoans is simple and does not include nephridia or other excretory organs. However, it is believed that nitrogenous waste such as ammonia diffuses out the body wall and lophophore. More-complex waste products are not excreted but accumulate in the polypide, which deteriorates over several weeks. Some of the old polypide is recycled, but much of it remains as a large mass of senile cells containing accumulated wastes, and this is compressed into a “brown body.” Once the degeneration is complete, the cystid (outer part of the organism) produces a new polypide, and the brown body remains in the coelomic cavity, or in the stomach of the new polypide, to be expelled the next time the individual defecates.
With regard to reproduction, bryozoan zooids of all phylactolaemate species are simultaneous hermaphroditic, and some species may produce sperm and eggs in some zooids, while other species have colonies with separate male and female zooids. Many marine species are protandric and function initially as males and then as females as their colonies contain a combination of zooids that are in their respective sexual stages. Cupuladriid bryozoans are capable of both sexual and asexual (clonal) reproduction.
Testes of bryozoans develop on the funiculus connecting the stomach to the body wall, while diffuse ovaries usually arise from translucent patches of the peritoneum at the cystid wall. Eggs and sperm are released into the coelom, and sperm exit into the water through pores in the tips of some of the tentacles and then are captured by the feeding currents of zooids that are producing eggs. Some species possess eggs that are fertilized externally after being released through a pore between two tentacles, which, in some cases, is at the tip of a small projection called the “intertentacular organ” in the base of a pair of tentacles. Conversely, others are fertilized internally, in the intertentacular organ or in the coelom. In ctenostomes, the mother provides a brood chamber for the fertilized eggs and her polypide disintegrates, providing nourishment to the embryo. Stenolaemates produce specialized zooids to serve as brood chambers, and their eggs divide within here to produce up to 100 identical embryos. Every colony begins from the tissues of a metamorphosed larva that develops one or a few zooids from the body wall, or from a mass of undifferentiated cells called a blastema. The first zooid or group of zooids is known as the ancestrula. The ancestrula is round rather than shaped like a normal zooid of the species. It undergoes budding to produce daughter zooids, which later form more buds, and so on. In addition to budding, freshwater bryozoans reproduce asexually by the formation of statoblasts that are externally resistant to drying and freezing and are produced in large numbers during bad environmental conditions.
The tentacle crown (lophophore) generally has a horseshoe-shaped appearance in phyactolaemates and is circular in the gymnolaemates. The tentacles are ciliated with microvilli and absorb organic compounds dissolved in the water. The digestive tract is U-shaped, and the mouth lies within the tentacle ring. Digestion begins extracellularly in the cardiac and central stomach and is completed intracellularly in all parts of the stomach. Food is moved along through the gut by cilia and peristalsis. Any undigested food is formed into a spindle-like mass and then passed to the rectum for passage.
Most bryozoans are filter feeders that sieve food particles (mainly phyto- and zooplankton and protists) out of the water column using the retractable lophophore, a crown of tentacles lined with cilia. Bryozoans capture various food particles with their tentacles, including microorganisms (bacteria, algae, protozoa, small nematodes, rotifers, and small crustaceans), but the primary assimilated food source is probably small (<5 μm diameter) bacteria and fungi attached to dead organic matter in suspension.
The living zooids of freshwater bryozoans are grazed upon by many predators, including snails, flatworms, oligochaetes, crayfish, aquatic insects (especially caddisflies and elmid beetles), and fish. Predators of marine bryozoans include crustaceans, orbatid mites, nudibranchs, pycnogonids, sea urchins, starfish, and fish. In general, marine echinoderms and mollusks eat masses of zooids by gouging pieces of colonies, whereas most arthropod predators eat individual zooids. In Thailand, the introduced golden apple snail (Pomacea canaliculata) has destroyed phylactolaemate populations wherever it has appeared. It has also been reported to prey on a common freshwater gymnolaemate, but with less devastating effect. Interestingly, native snails do not feed on bryozoans. Bryozoan statoblasts are often found among the stomach contents of fish, suggesting a certain degree of predation. Interestingly, fredericellid bryozoans (Fredericella sultana) are reported to compose as much as 75% by volume of the three species of sunfish (Lepomis spp.) diet at Bull Shoals Reservoir. In addition, bryozoans made up 35% of the diet of bluegill (L. macrochirus) in DeGray Reservoir.
Symbiotic relationships have been reported among several species of hydroids (family Zancleidae) with bryozoans, some of which are beneficial to the hydroids, while others are parasitic. One bryozoan, Alcyonidium nodosum (order Ctenostomata), appears to protect the whelk (Burnupena papyracea) against predation by the rock lobster (Jasus lalandii). While encrusted whelk shells by the bryozoans are stronger than those without this reinforcement, chemical defenses produced by the bryozoans are probably the more significant deterrent. Another bryozoan, Acanthodesia commensale, has formed a non-obligate symbiotic relationship with some hermit crabs in egg-size structures known as bryoliths.
In some hatcheries and fish farms, salmonid stock have been lost to proliferative kidney disease, caused by one or more myxozoans (particularly Tetracapsuloides bryosalmonae) that utilize bryozoans as alternate hosts. Some fishermen in the North Sea complain about a form of skin disorder (eczema) known as “Dogger Bank itch,” thought to be caused by contact with bryozoans that have stuck to nets and lobster pots. Because they are among the first colonizers of new or recently cleaned structures, marine bryozoans are often responsible for biofouling on ships’ hulls, on docks and marinas, and on offshore structures. Freshwater species are occasional nuisances in water pipes, drinking-water purification equipment, sewage treatment facilities, and the cooling pipes of power stations.
Some interesting bryozoans include Membranipora tuberculata, an encrusting marine species with zooids arranged like fine brickwork; Bugula neritina, an erect marine branching colony that resembles seaweed; and Plumatella repens, a freshwater species that grows on the underside of rocks and vegetation in lakes, ponds, and streams. Another noteworthy freshwater bryozoan is the Pectinatella magnifica (class Phylactolaemata, order Plumatellida). Joseph M. Leidy (1823‒1891) described P. magnifica in 1851 from near Philadelphia. It is native to freshwater drainages of eastern North America from New Brunswick and Ontario, Canada, and south to Florida and Mississippi. This bryozoan is invasive in parts of Europe and northern Asia. Zooids of P. magnifica live directly on submerged substrata in a colony. However, unlike other freshwater bryozoans, it makes its own substrate, forming large gelatinous colonies that grow attached to submerged surfaces such as a rock or branch. In some areas, it is widely distributed and common but often overlooked because most colonies are small and unremarkable. In the southeastern United States, large (30 to 60 cm [11.8 to 23.6 in.], in diameter) gelatinous colonies of P. magnifica are a common and impressive sight in some lakes and reservoirs and occasionally in streams and ponds where they are sometimes called “dinosaur snot.” Large colonies of this species are common in Lake Wilhelmina in Polk County, and some have been reported at a pond in Elm Park, just off of State Highway 23 in Scott County and the Dale Bumpers White River National Wildlife Refuge (most in eastern Arkansas County). Also, their presence in watersheds usually indicates good water quality, but some species tolerate eutrophic conditions and organically polluted waters.
The International Bryozoology Association (IBA), founded in 1965, is a scientific society with international membership specializing in research of bryozoans.
For additional information:
Arbuckle, Karen. “Hypothetical Origins of Paleozoic Fossils from the Gulf Coastal Plain of Arkansas.” Journal of the Arkansas Academy of Science 54 (2000): 19‒23. Online at http://scholarworks.uark.edu/jaas/vol54/iss1/5 (accessed December 5, 2019).
Banta, W. C., J. D. Soule, and D. F. Soule. “Preparing Study Specimens of Hard Parts of Recent Bryozoa.” Chesapeake Science 14 (1973): 62‒64.
Brusca, R. C., W. Moore, and S. M. Schuster. Invertebrates. 3rd ed. Sunderland, MA: Sinauer Associated Incorporated Publishers, 2018.
Bryant, Horace E., and Thomas E. Moen. “Food of Bluegill and Longear Sunfish in DeGray Reservoir, Arkansas, 1976.” Proceedings of the Arkansas Academy of Science 34 (1980): 31‒33. Online at https://scholarworks.uark.edu/jaas/vol34/iss1/10 (accessed December 5, 2019).
Chordas, Stephen W. III, George L Harp, and G. W. Wolfe. “Aquatic Macroinvertebrates of the White River National Wildlife Refuge, Arkansas.” Proceedings of the Arkansas Academy of Science 50 (1996): 42‒51. Online at http://scholarworks.uark.edu/jaas/vol50/iss1/9 (accessed December 5, 2019).
Dendy, J. S. “Observations on Bryozoan Ecology in Farm Ponds.” Limnology and Oceanography 8 (1963): 478‒482.
Girty, George H. “The Fauna of the Moorefield Shale of Arkansas.” U.S. Geological Survey Bulletin 439 (1923): 1‒148.
Greenland, D. C., S. H. Newton, and R. F. Faucette Jr. “Effects of Cage Encrustation by the Bryozoan Plumatella casmiana on Production of Channel Catfish.” Progressive Fish Culturist 50 (1988): 42‒45.
Hyman, Libbie H. The Invertebrates: Smaller Coelomate Groups. Vol. 5. New York: McGraw-Hill, 1959.
Joo, G., A. K. Ward, and G. M. Ward. “Ecology of Pectinatella magnifica (Bryozoa) in an Alabama Oxbow Lake: Colony Growth and Association with Algae.” Journal of North American Benthological Society 11 (1992): 324‒333.
Massard, J. A., and G. Geimer. “Global Diversity of Bryozoans (Bryozoa or Ectoprocta) in Freshwater: An Update.” Bulletin de la Société des Naturalistes Luxembourgeois 109 (2008): 139–148.
Matsuyama, Kei, Jürgen Titschack, Daniel Baum, and André Freiwald. “Two New Species of Erect Bryozoa (Gymnolaemata: Cheilostomata) and the Application of Non-Destructive Imaging Methods for Quantitative Taxonomy.” Zootaxa 4020 (2015): 81‒100.
Mayr, Ernst. “Bryozoa Versus Ectoprocta.” Systematic Zoology 16 (1968): 213‒216.
McKinney, Frank K., and J. B. C. Jackson. Bryozoan Evolution. London: Unwin Hyman, 1989.
Neck, Raymond, and Richard Fullington. “New Records of the Freshwater Ectoproct Pectinatella magnifica in Eastern Texas.” Texas Journal of Science 35 (1983): 269–271.
Nielsen, C., and K. Worsaae. “Structure and Occurrence of Cyphonautes Larvae (Bryozoa, Ectoprocta).” Journal of Morphology 271 (2010): 1094‒1109.
Okamura, B., and T. S. Wood. “Bryozoans as Hosts for Tetracapsula bryosalmonae, the PKX Organism.” Journal of Fish Diseases 25 (2002): 469‒475.
Pennak, Robert W. Fresh-Water Invertebrates of the United States. 3rd ed. New York: John Wiley & Sons, 1989.
Rubini, A., G. Pieroni, A. Elia, L. Zippilli, F. Paolocci, and M. Taticchi. “Novel Morphological and Genetic Tools to Discriminate Species among the Family Plumatellidae (Phylactolaemata, Bryozoa).” Hydrobiologia 664 (2011): 81–93.
Taylor, Paul D., and Frank K. McKinney. “Reinterpretation of Stictostega Shaw, 1967, an Upper Cretaceous Cheilostome Bryozoan from Arkansas.” Journal of Paleontology 74 (2000): 1‒6.
Thorp, J. H., and A. P. Covich, eds. Ecology and Classification of North American Freshwater Invertebrates. San Diego, CA: Academic Press, 1991.
Wilson, Mark A., and Paul D. Taylor. “A New Runner-like Cyclostome Bryozoan from the Bromide Formation (Sandbian, Upper Ordovician) of Oklahoma and its Phylogenetic Affinities.” Journal of Paleontology 90 (2016): 413‒417.
Winston, J. E. “Polypide Morphology and Feeding Behavior in Marine Ectoprocts.” Bulletin of Marine Science 28 (1978): 1‒31.
Wood, Timothy S. “Bryozoans.” In Ecology and Classification of North American Freshwater Invertebrates, edited by James H. Thorp and Alan P. Covich. London: Academic Press, 2009.
———. “The Pipeline Menace of Freshwater Bryozoans.” Denisia 16 (2005): 203–208.
———. “Study Methods for Freshwater Bryozoans.” Denisia 16 (2005): 103–110.
Henry W. Robison
Sherwood, Arkansas
Chris T. McAllister
Eastern Oklahoma State College
 Science and Technology
Science and Technology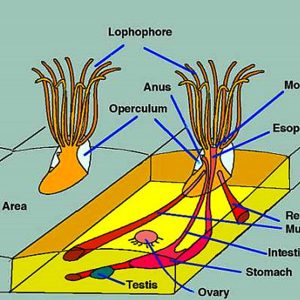 Bryozoan Morphology
Bryozoan Morphology  Various Bryozoans
Various Bryozoans  Fossilized Bryozoans
Fossilized Bryozoans  Pectinatella magnifica
Pectinatella magnifica 




Comments
No comments on this entry yet.