1. Introduction
Armeria maritima (
A. maritima) belongs to the
Armeria Willd. genus and the
Plumbaginaceae L. family. The
Armeria genus has about 164 species spread across all continents except Australia and Antarctica. There are several dozen species of the
Armeria genus in Europe. The homeland of
Armeria in Europe is the Mediterranean region
[1][2]. The
A. maritima species is extraordinarily variable, as demonstrated by its morphology. Even though the subspecies are discerned based on their characteristic traits, their differences are often blurry, which will be discussed in more detail later. The individual subspecies also differ in their geographical distribution and habitat preferences
[1][2][3][4][5].
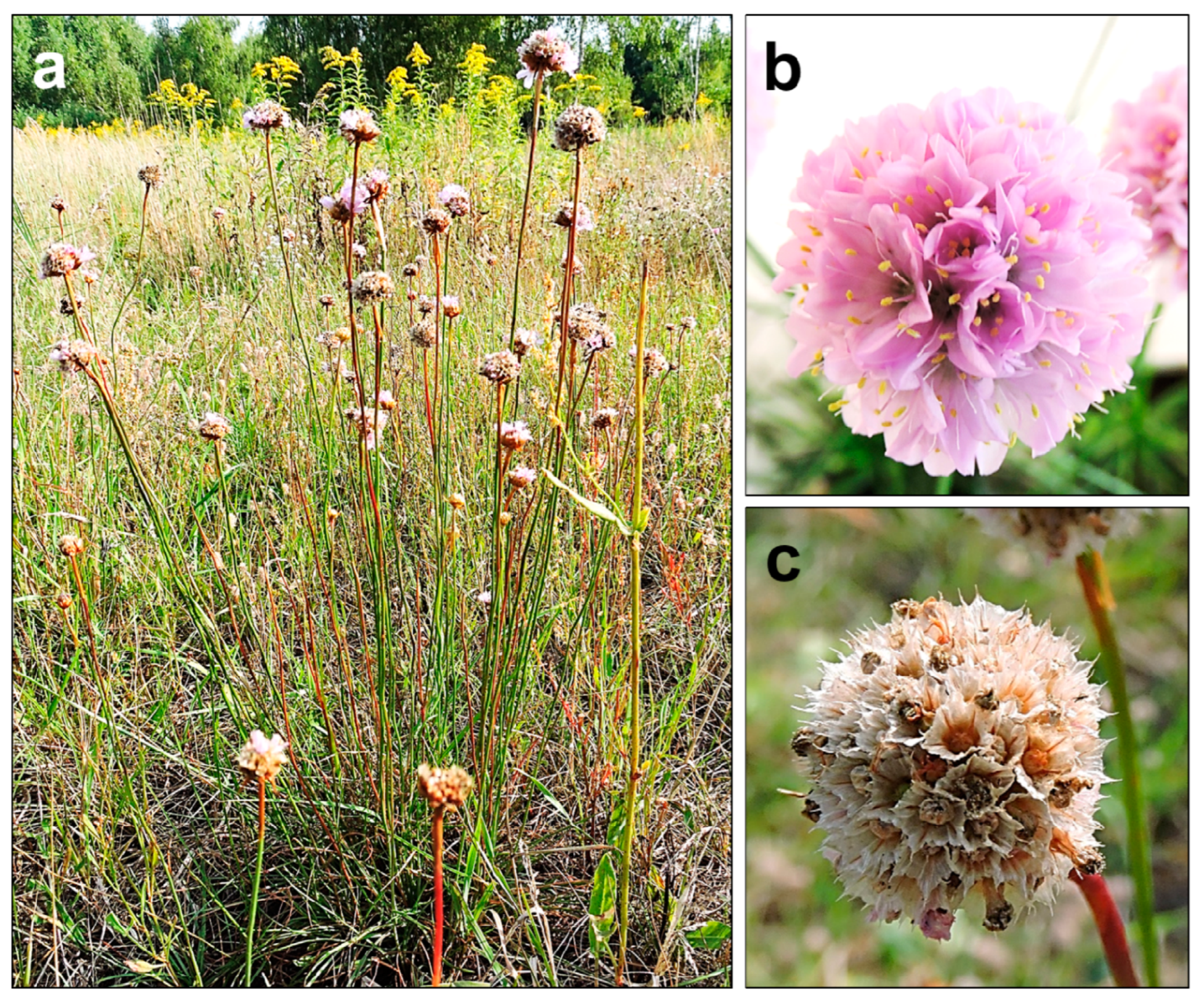
A. maritima is a perennial herb, classified by the Raunkiaer’s system as a hemicryptophyte—an earth-bud plant whose perennating buds are formed just below the soil surface. This species is characterized by single, evenly narrow, elongated, pointy, or blunt leaves about 2 mm wide. The leaves are gathered in a basal rosette, and an erect leafless shoot (scape) bears a single head-shaped inflorescence. The leaves are slightly pubescent, especially around the edges (less often glabrous). The tap root can reach 1.5 m in length and can have many small divaricate roots in the upper 20–30 cm of soil. The scape is 30–50 cm long, and slightly pubescent or glabrous. An individual usually has several inflorescence shoots (
Figure 1a). On average, the inflorescences are approximately 12 mm in diameter. They are composed of radiant five-fold flowers, colored in shades of pink (from almost white to occasionally deep purple-red) (
Figure 1b). At the base of the inflorescence head, there is a tubular scarious sheath. The inflorescence bud is supported by light green, scarious outer and inner involucral bracts. Each flower has five stamens (their filaments are 5–8 mm and broadened below), joined to the base of the petals, and five styles (filiform, free to near the base, pubescent below). The flowering period lasts from May to October.
A. maritima has a nut-like, single-seeded fruit (about 2 mm long) dehiscing transversely above or irregularly below. The fruit is enclosed in the calyx (
Figure 1c), which is tubular below and extended above into a persistent scarious pleat. During fruiting, it reaches a length of 5–7.5 mm. The fruit weights range from about 0.7 to 1 mg. Each subspecies has some unique features. However, their distinction is not always possible due to the high morphological plasticity
[1][4][5][6][7][8][9].
Figure 1. The appearance of A. maritima plants from the area near Warsaw (Poland), not contaminated with heavy metals: (a) habitat of the whole plant; (b) inflorescence with developed flowers; (c) seeds in calyxes. All photos by Olga Bemowska-Kałabun.
The reproductive biology of
A. maritima has been the subject of many studies.
A. maritima individuals grow singly or form a compact turf by producing daughter rosettes on a joint vegetative shoot
[3]. According to Lefèbvre (1976)
[10], the heteromorphic self-incompatibility (due to dimorphic surface features of the pistil and pollen grains) occurring in
A. maritima causes its strict allogamy. Eisikowitch and Woodell (1975)
[11] also claimed that
A. maritima is a dimorphic self-incompatible perennial plant. However, there are contradictory reports on the possibility of self-fertilization in this species. Philipp et al. (1992)
[12] have not found any evidence of this phenomenon, whereas others
[10][13][14][15][16] have recorded self-compliant (self-pollinating)
A. maritima individuals among its metallicolous populations. According to Lefèbvre (1976)
[10], the possibility of self-pollination in
A. maritima may depend on its origin and even the way it is pollinated. Here, researchers question what pollinators does
A. maritima have? For example, Eisikowitch and Woodell (1975)
[11] indicated
Bombus lucorum and
B. terrestris as the primary pollinators of
A. maritima subsp.
maritima on dunes and shingles in Britain. In general, it is the common bee that frequently pollinates
A. maritima [11].
A. maritima inhabits much of the northern hemisphere. The species is common across most of continental Europe, except for the eastern region. The range of
A. maritima subsp.
maritima, an obligatory halophyte occurring only in saline areas, includes the Baltic, North Sea, and Atlantic coasts, is a dominant subspecies in Western Europe. Less commonly distributed is
A. maritima subsp.
elongata, with its range stretching over Central and Eastern Europe.
A. maritima subsp.
halleri, with its disjunctive range covering only metalliferous regions, is the taxon spanning the narrowest range. This subspecies is an obligatory metallophyte and is regarded as an indicator of copper, zinc, and lead ore-bearing areas. It is common in the regions where non-ferrous metal ores occur and are processed
[2][3][5][17][18].
A. maritima occurs in grassland communities, dry grasslands on sandy soils, as well as areas with specific habitat conditions (mountain, saline, and metalliferous regions). Generally, the species prefers mineral-humus, fresh, and moderately poor (mesotrophic) soils with neutral pH (about 6–7), which corresponds with the conditions found on waste heaps in three metalliferous areas (Poland, France, and Belgium), as described in the previous chapter. The species occurs in such phytocoenoses as
Vicio lathyroidis-Potentillion argenteae,
Diantho-Armerietum elongate, and
Corynephoro-Silenetum tataricae [1][2][3][5][8][18][19][20][21].
A. maritima is characterized by a broad ecological amplitude and may settle in natural wild or extremely harsh environments, such as the zinc–lead waste heaps. Given the robust nature of this species, the adaptations
A. maritima has developed to live in metalliferous areas are worth examining.
2. Armeria maritima Adaptations to Heavy Metals
To accurately study the adaptation mechanisms in
A. maritima, the research was conducted under controlled laboratory conditions. Multiple research techniques—including morphology, cytology, electron microscopy, and molecular biology—were used to perform experiments and compare results. The research on the
A. maritima adaptations to heavy-metal-enriched habitats has revealed a complex network of mechanisms working in unison to keep the plant in good condition. Studies show that
A. maritima specimens from metalliferous areas differ in their morphological features, tolerance levels, and development degrees of resistance mechanisms to heavy metals compared to their counterparts growing in non-metalliferous areas. For example, Olko et al. (2008)
[6] and Abratowska et al. (2015)
[17] showed that
A. maritima populations from a metalliferous area (zinc–lead waste heap in Bolesław, Poland) and non-metalliferous area (a rural area in southern Poland) differed in their tolerance to zinc, lead, and cadmium. Plants from the metalliferous population showed a higher tolerance to these metals by about 20% in the Wilkins’ root tolerance tests compared to plants from the non-metalliferous area. In this section, the most advanced adaptations of
A. maritima to heavy metals will be discussed, and the differences in metal protective responses resulting from a microevolution process between plants from metalliferous and non-metalliferous areas will be presented.
3. Avoidance and Tolerance Mechanisms
Before discussing
A. maritima’s adaptations to growth in a metalliferous area, it is essential to differentiate the general protective responses of plants to heavy metals. These mechanisms are divided into two groups: (1) avoidance of heavy metals, which prevents metals entering ions into a cell; (2) tolerance of heavy metals, which is a response to the presence of metal ions in a cell
[22][23][24][25].
Plants deploy the following avoidance strategies:
-
Exclusion—processes that prevent the absorption of heavy metals by a plant, e.g., the release of metal-ion-chelating compounds into the rhizosphere, via which metals become immobilized or captured in roots and shoots, preventing the spread of metals in a plant;
-
Elimination—processes that work after heavy metals have entered a plant’s tissues and lead to the expulsion of metals to the outside, e.g., heavy metals are excreted through the plant surface, secreted by glands and trichomes, or the entire organs with the heavy metal content, such as the oldest leaves, wither and fall off;
-
Redistribution—processes that reduce the presence of heavy metals and transport them to sections of the plant, where there is a lower risk of their toxic effects on an organism, such as aging leaves; this process works in combination with elimination;
-
Compartmentation—a process that occurs at the cellular level, wherein heavy metals accumulate in regions of the plant where they no longer threaten the cells, e.g., in cell walls, intercellular spaces, and vacuoles.
In turn, the tolerance mechanisms work when heavy metal ions have already entered the cell and pose a threat to cellular metabolism. In response to an increase in metal concentration in the cytoplasm, stress proteins (also synthesized in response to other stressors), polypeptides, and stress metabolites are secreted, e.g., heat shock proteins (HSP) and ras-associated binding proteins (RAB), osmotins, proline, metal chelators such as glutathione or phytochelatin, amino and organic acids, signal transducers, structural proteins, enzymes, and many others
[22][23][24][25][26][27][28][29][30][31][32][33].
4. Armeria maritima Adaptation to Heavy Metals at the Levels of the Whole Organism, Individual Tissues, and Cells
The most important resistance strategy of
A. maritima at the organism level was the retention of heavy metals (lead, cadmium, zinc, and copper) in the roots and the metal accumulation in the oldest withering leaves, as rejected by the plants (redistribution and elimination, also associated with compartmentation and exclusion). This mechanism limits the transport of metals to green leaves and generative organs. Interestingly, these strategies are common to plants growing in contaminated areas (generation F0) and those cultivated with heavy metals in laboratory conditions (generation F1).
A. maritima plants cultivated experimentally in mineral media were characterized by a similar distribution of metals as plants growing in outdoor conditions in metalliferous areas. This observation indicates the genetic fixation of the ability to defend against heavy metals in
A. maritima plants from metalliferous sites. Moreover, cultivated under laboratory conditions, plants from metalliferous and non-metalliferous populations showed the same metal accumulation pattern, indicating that this is a feature inherent to the
A. maritima species
[3][6][19][24][26][34][35][36][37]. The subsequent sections describe these protective responses in more detail.
The research on
A. maritima showed zinc accumulation in the outer layers of the root, more specifically the rhizoderma and cortical cells, concentrated mainly in the cell walls and vacuoles (compartmentation mechanism)
[24][37]. Heavy metals are transported in these layers by the apoplastic route up to the innermost cortex, the endoderm. The endoderm is a single layer of cells. Suberinic thickening, the so-called Caspary’s strand, surrounds each of those cells and stops the apoplastic radial transport. The only way substances may penetrate further is via the symplast of the endodermal cells. This suberinic barrier is very effective in blocking the transport of heavy metals deep into the root and preventing their movement to the aboveground parts of the plant
[22][24][34][37][38]. Some research has shown that the endoderm of the
A. maritima root retains zinc by limiting its transport to the aboveground parts
[24][37]. Szarek et al. (2004)
[34], who studied the metal accumulation in
A. maritima from metalliferous and non-metalliferous soils in laboratory conditions, found significant differences in the concentrations of lead, zinc, and cadmium between the roots and green leave. This result demonstrated the limited transport of metals from the roots to aboveground parts of
A. maritima. This is an example of an exclusion strategy
[34]. Similarly, Brewin et al. (2003)
[39] showed that
A. maritima roots and living leaves generally act as copper excluders, whereas decaying leaves act as copper accumulators. The authors pointed out that the higher copper retention in the roots compared to the living leaves and copper excretion through the decaying leaves point to different mechanisms of copper tolerance
[39]. The work by Dahmani-Muller et al. (2000)
[19] showed that in
A. maritima subsp.
halleri, the lead and copper concentrations were 20 and 88 times higher, respectively, in the roots than in the green leaves; the authors suggested an exclusion strategy by metal immobilization in the roots. Relevant to this matter is the study by Neumann et al. (1995)
[36], who showed that
A. maritima subsp.
halleri accumulates copper in the vacuoles of the tannic root cells (also known as idioblasts) via chelation with polyhydroxy phenolic compounds; this issue will be discussed in more detail in later sections of the review. Dahmani-Muller et al. (2000)
[19] suggested that such metal–phenol complexes are transported from the roots to aging leaves, which brown and fall. Ernst et al. (1992)
[40] also proposed leaf shedding as a metal detoxification mechanism
[19].
The characteristic trait of
A. maritima is the intensified metal accumulation in the oldest leaves compared to the green ones. Inhibiting the transport of metal ions to green leaves and generative organs protects plants’ most sensitive processes, i.e., photosynthesis and reproduction, against the toxic effects of heavy metals
[3][19][34]. Dahmani-Muller et al. (2000)
[19] showed that the zinc, cadmium, lead, and copper concentrations in the brown leaves of
A. maritima subsp.
halleri from northern France were 3–8 times higher than in green leaves. The authors linked the subsequent leaf fall to the detoxification mechanism
[19]. In turn, the lead concentration in the oldest leaves of the
A. maritima plants growing on the waste heaps in Bolesław (the Olkusz region in Poland) and the Plombières region exceeded the hyperaccumulation level, i.e., it surpassed 1000 mg/kg DW
[3][17].
The plants cultivated in laboratory conditions—in mineral solutions enriched with metals—were characterized by similar metal distributions as the plants growing on waste heaps. Moreover, the experiments under laboratory conditions showed that the metal accumulation process was the same in the plants from metalliferous and non-metalliferous populations
[3][17][34]. Dahmani-Muller et al. (2000)
[19] suggested that the translocation of metals to senescent leaves in the
A. maritima plants might be an active process assisted by phenolic compounds, which the tissues of
A. maritima plants are rich in
[19]. The metal quantity accumulated in the tissues of these plants was very high, exceeding the lethal level for most ‘ordinary’ plants. This accumulation pattern may be a specific trait of the A. maritima species.
How does
A. maritima transport metal elements around its cellular body? Their transport method in leaves from the vascular bundle to the epidermis passes through the mesophyll—a tissue composed of photosynthetically active cells—is the most sensitive to the toxic effects of metals. Therefore, metal transport must involve both the apoplastic and symplastic pathways. In the
A. maritima plants, unique mechanisms prevent the harmful effects of excess metal in mesophyll
[3][17][34]. The research on lead transport in the
A. maritima cut-off leaves showed that the main lead accumulation areas were vascular bundles, mesophyll cell walls (primarily those located close to the bundle), and intercellular spaces. Only a small amount of lead penetrated the cells. Other data showed that lead, cadmium, and zinc were found mainly in vascular bundles and apoplasts of the mesophyll area. Thus, one of the detoxification mechanisms in the mesophyll region is to bind heavy metals in cell walls and intercellular spaces (the compartmentation mechanism)
[17][24]. Additionally, Heumann et al. (2002)
[37] found zinc grains in the leaves of
A. maritima subsp.
helleri. Specifically, these grains localized extracellularly to all cell walls and intracellularly to xylem vessels, vacuoles of transfer cells, parenchyma cells, gland cells, and plasmodesmata of various cell types. In general, the zinc found in those leaves accumulated in the cell walls of vascular bundles and the cell walls and vacuoles of mesophyll cells
[37]. Substantial metal accumulation was also found in and around the secretory cells, as discussed in the subsequent paragraphs. Other authors observed similar phenomena
[6][17][24][37]. For example, Szarek-Łukaszewska (2004)
[34] found the largest concentrations of heavy metals in withering leaves in vascular tissues and surrounding parenchymal cells. She also detected metals in the parenchyma and epidermis cell walls, coinciding with the results described in this paragraph.
Moving metals to sections of the plant where they do not pose a direct hazard to metabolism is one of the plants’ most essential detoxification mechanisms
[30][41]. An interesting avoidance mechanism against heavy metals in the
A. maritima plants relates to the functioning of the epidermal structures—the trichomes and salt glands—an example of the elimination mechanism
[3][6][17]. The epidermis and its structures, like trichomes, are the target places to accumulate metals in leaves. The epidermal cells, except for the stomatal guard cells, are characterized by lower metabolic activity than the mesophyll cells. The epidermal structures may, therefore, accumulate the excess of metals, lowering their concentration in the mesophyll cells of leaves. Consequently, the epidermal structures protect sensitive physiological processes
[32]. However, whether the mechanisms related to the functioning of epidemic structures are more efficient in plants from metalliferous than non-metalliferous sites awaits confirmation.
According to various authors, heavy metals such as lead, cadmium, zinc, and copper accumulate in the trichomes and salt glands of the
A. maritima leaf epidermis
[3][6][17][37]. For example, the metals accumulate in the epidermal trichomes after the cultivation of the
A. maritima plants with the addition of lead, cadmium, and zinc. The metals localize the cell walls of trichomes, especially at their base and on the inside of the cells. Data show that
A. maritima plants from metalliferous and non-metalliferous populations rely on this mechanism
[17][24]. The study by Olko et al. (2008)
[6] found the highest metal concentrations in the leaf epidermal and subepidermal tissues, including trichomes. Thus, trichomes may play an important role in heavy metal storage. Similar results, showing metal accumulation in epidermal structures, including trichomes, were also obtained for other metallophytes, including
S. vulgaris [42],
T. caerulescens [33][43],
A. halleri [44][45], or
B. laevigata [22][46]. In the cited studies, metal accumulation in the epidermal cells and trichomes was considered one of the most critical mechanisms of hyperaccumulation. The objective of other studies using different species was also to explore the mechanism of metal accumulation in trichomes. For example, in the
Vicia faba epidermal trichome cells, the metal action increased the expression of metallothioneins
[47]. On the other hand, in the
A. halleri trichomes, there was a higher level of glutathione and increased expression of genes encoding its biosynthesis
[48]. Further research on the mechanism of metal accumulation in the
A. maritima trichomes is still needed.
Another feature of
A. maritima is the heavy metal secretion by salt glands
[3][6][17][36][37][49][50][51]. These specialized secretory structures are formed by the leaf epidermis. They play a key role in regulating salt concentrations in halophytes that grow on saline substrates. The saline solution concentrates inside the secretory cells of the gland and travels to the zone between the cellulosic part of the cell wall and the cuticle layer lifted over the gland. The solution then drains through the pores in the cuticle. The secretory cells differ in their ultrastructure from other epidermal cells. The difference, mainly in the bigger number of mitochondria, indicates that salt secretion is an active process requiring an energy supply. A thick layer of cutin that saturates the cell walls separates the secretory cells from the mesophyll cells. This barrier prevents a backflow of the concentrated salt solution to the mesophyll
[52][53][54]. There is also a hydrophobic substance between the secretory cells and the internal cells at the base of the salt gland, which does not saturate the cell wall but adheres to it. As a result, a continuum of the apoplast between the mesophyll cells and the cells of the gland base is preserved
[54].
Naturally,
A. maritima often grows in highly saline soils, e.g., coastal areas. The salt glands are found in its leaves’ upper and lower epidermis. A cuticular layer surrounds them with pores at the leaf surface and cutin-free transfusion zones, where outer basal cells are contiguous with surrounding sub-basal cells. The salt glands are built from sixteen cells (arranged into four quadrants). Four secretory cells, located in the inner quadrant, are surrounded apically by four accessory cells. Four inner and four outer basal cells cover the secretory and accessory cells. All of the cells have a very dense cytoplasm rich in organelles. They also have more or less developed wall protuberances. In the
A. maritima plants growing in saline areas, the action of salt glands is an important immune mechanism that allows the removal of excess salt from leaf tissues
[1][8][37]. Does the appearance of salt glands differ between subspecies? According to Heumann (2002)
[37], the upper and lower leaf epidermal tissues of
A. maritima subsp.
halleri possess specialized multicellular salt-secreting glands. Their structure is identical to that in the halophyte,
A. maritima subsp.
Maritima, and other salt-tolerant
Plumbaginaceae [37]. In turn, Neumann et al. (1995)
[36] showed that
A. maritima subsp.
halleri growing on non-contaminated soil displays the same morphologic structure of leaf salt glands as plants from the mine mound in the medieval copper mining region in Germany. Thus, the salt glands appear the same within the whole
A. maritima species
[36][37]. Several studies indicate that the salt glands are involved in metal detoxification in the
A. maritima plants growing in metalliferous areas. The removal of heavy metals through salt glands is one of the species’ adaptations, but its mechanism is still not fully understood
[3][6][17][36][37][49][50][51]. For example, salt crystals covered the leaves of the
A. maritima plants during cultivation in a substrate supplemented with lead, cadmium, and zinc. The three metals given to the plants were found in the epidermal salt gland cells and the salt content secreted onto the leaf surface. They were, thus, secreted onto the leaf surface by the salt glands. The secreted solution also contained other elements and mineral components of the cultivation substrate, including calcium, sulfur, chlorine, magnesium, and potassium. Therefore, the regulation of the metal concentration in the leaf mesophyll depended on the salt glands. This mechanism occurred in plants from metalliferous (e.g., zinc–lead heaps) and non-metalliferous regions
[3][17][51].
5. Armeria maritima Adaptation to Heavy Metals at a Physiological and Biochemical Level
The production of glutathione (GSH), phytochelatins (PCs), and organic acids in plants is a manifestation of tolerance mechanisms. These compounds maintain intracellular homeostasis by detoxifying metal ions and protecting against their toxic effects. Glutathione is an antioxidant. An increase in its amount protects cells against oxidative stress. Phytochelatins formed from glutathione with the phytochelatin synthase activity bind free metal forms within the cytosol and participate in intracellular metal transport. Organic acids participate in the long-distance transport of metals and bind metals within a vacuole, playing a detoxification function inside a cell
[6][22][34][55][56][57][58][59].
Growth adaptations under excess toxic heavy metals were also investigated at the physiological and biochemical levels of the
A. maritima plants. No phytochelatins were found in the
A. maritima plants cultivated with zinc, lead, and cadmium
[6]. However, the authors of the study observed an increase in the glutathione content and changes in the content and proportions of organic acids, such as malic acid. The glutathione pool increased in the plants from the zinc–lead waste heap in Bolesław (near Olkusz in Poland) and in those from the non-contaminated area. Additionally, they found a change in the malic acid levels in plants from the zinc–lead waste heap population. The contents decreased in the roots but increased significantly in the leaves. Such a high increase in the malic acid content in the leaves indicated its intensified transport and the synthesis of additional pools in those leaves
[3][6][17].
Another tolerance mechanism in the
A. maritima plants is related to the presence of the so-called tannin cells containing phenolic compounds in vacuoles. These cells occur abundantly throughout the tissues of this species. In the
A. maritima plants growing on soils enriched with zinc and copper, the tannin cells accumulate metals
[36][37]. Neumann et al. (1995)
[36] showed that in the roots of
A. maritima, the multiseriated sclerenchymatous exodermis contains various types of idioblasts. In the cortical parenchyma, idioblasts (which included the vacuolar clusters and osmiophilic material between the cell walls and the plasmalemma) were often found. This research also showed that many idioblasts with osmiophilic precipitates in the vacuoles occur in the leaves, their epidermis, the palisade parenchyma, and the spongy parenchyma—around and inside the bundle. A similar structure was found in plants from metalliferous (a medieval copper mining region in Saugrund-Eisleben, Germany) and non-metalliferous areas (the Botanical Gardens, Halle, Germany). In the roots of
A. maritima, the vacuoles of numerous idioblasts turned out to be the primary storage compartments for copper
[36]. Other studies showed that during plant cultivation in the lead-, cadmium-, and zinc-enriched medium, phenolic compounds in the vacuoles of the root tannin cells frequently bound the heavy metals
[3][17]. The
A. maritima population from the metalliferous area (a waste heap in Bolesław, Poland) showed a higher tolerance level to lead, cadmium, and zinc than the population from the non-contaminated site. In the roots of
A. maritima from the contaminated area, the number of tannin cells was higher than in the more heavy-metal-sensitive population from the uncontaminated area. The vacuoles of these cells contained phenolic compounds with heavy metal deposits. The deposits comprised lead, cadmium, or zinc, depending on which metals were given to the plants during growth. Therefore, the protective action of phenolic compounds was a critical feature responsible for increasing the tolerance level to the metals in the
A. maritima plants from zinc–lead waste heaps
[3][17]. For comparison, Heumann (2002)
[37] found in the roots of
A. maritima from the metalliferous area (near a lead smelter at Stolberg, Germany) zinc-enriched granules—extracellularly in the cell walls of the rhizodermal and outer cortical cells, all the way to the anticlinal walls of the endodermis. Similar deposits were also found intracellularly in the vacuoles of rhizodermal cells, the outer cortical cells, the endodermis cells, and the xylem vessels. The zinc granules were also found in leaves extracellularly in all cell walls; intracellularly in the xylem vessels, vacuoles of transfer cells, parenchyma, and gland cells; and in plasmodesmata between distinct cell types
[37]. Therefore, in addition to the vacuoles, the cell walls are also important sites for metal detoxification in this species.
The last physiological–biochemical adaptation to the excess of heavy metals was found in the
A. maritima plants growing in the region of the former copper mine in Germany. The cytosol of the root cells of these plants contains the HSP17 low molecular weight stress protein. In contrast, HSP17 was not found in the leaves
[36]. HSP17 belongs to the heat shock proteins, formed not only at high temperatures but also engaging in heavy metal tolerance. They participate in the repair of intracellular damage caused by metals. HSPs function as chaperones in protein folding and assembly and can also protect and repair proteins damaged by oxidative stress caused by metals
[17][22][23][36][60].