Eurasian Treecreeper Certhia familiaris Scientific name definitions
Text last updated February 6, 2013
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Albanian | Piku rrotullues |
| Armenian | Ծվծվիկ |
| Asturian | Esguilñn nortiegu |
| Azerbaijani | Adi süzər |
| Basque | Basoetako gerri-txoria |
| Bulgarian | Горска дърволазка |
| Catalan | raspinell pirinenc |
| Chinese (SIM) | 旋木雀 |
| Croatian | kratkokljuni puzavac |
| Czech | šoupálek dlouhoprstý |
| Danish | Træløber |
| Dutch | Taigaboomkruiper |
| English | Eurasian Treecreeper |
| English (India) | European Treecreeper |
| English (United States) | Eurasian Treecreeper |
| Faroese | Trætølpur |
| Finnish | puukiipijä |
| French | Grimpereau des bois |
| French (France) | Grimpereau des bois |
| Galician | Subidor norteño |
| German | Waldbaumläufer |
| Greek | Βουνοδεντροβάτης |
| Hebrew | טפס-עצים אירופי |
| Hungarian | Hegyi fakusz |
| Icelandic | Skógfeti |
| Italian | Rampichino alpestre |
| Japanese | キバシリ |
| Korean | 나무발발이 |
| Latvian | Mizložņa |
| Lithuanian | Miškinis liputis |
| Mongolian | Хөвчийн бялзуумар |
| Norwegian | trekryper |
| Persian | دارخزک |
| Polish | pełzacz leśny |
| Portuguese (Portugal) | Trepadeira-do-norte |
| Romanian | Cojoaică de pădure |
| Russian | Обыкновенная пищуха |
| Serbian | Kratkokljuni puzić |
| Slovak | kôrovník dlhoprstý |
| Slovenian | Dolgoprsti plezalček |
| Spanish | Agateador Euroasiático |
| Spanish (Spain) | Agateador euroasiático |
| Swedish | trädkrypare |
| Turkish | Orman Tırmaşıkkuşu |
| Ukrainian | Підкоришник звичайний |
Certhia familiaris Linnaeus, 1758
Definitions
- CERTHIA
- certhia
- familiare / familiaris
The Key to Scientific Names
Legend Overview
Field Identification
12·5 cm; 7·8–10 g. Nominate race in fresh plumage (from Sept) has crown and nape bright rufous-brown, feathers narrowly fringed black and with white shaft streaks, lores blackish-rufous, long white supercilium from base of upper mandible to nape (may be indistinct over lores); cheek and ear-coverts mottled black, white and rufous-brown; mantle and scapulars similar or slightly warmer brown, feathers with prominent white centres and black tips (spotted and scalloped pattern), back, rump and uppertail-coverts contrastingly rufous, feathers with white centres; upperwing-coverts dark brown, lesser coverts with broad rufous-brown centres and darker tips, medians tipped whitish and broadly fringed rufous-brown, greaters broadly tipped off-white on outer web and fringed rufous-buff at base (may have fine white shaft streaks); alula dark brown, broadly tipped off-white; primary coverts dark brown, tipped pale buff on outer webs; inner webs of tertials pale grey-brown, outer webs brown but becoming black towards tip, fringed and broadly tipped whitish; primaries and secondaries dark brown, all of these except outer three primaries with broad creamy-buff band across feather (only on outer web on P4, primaries numbered ascendently), bordered on each side by black-brown band, bases of flight-feathers fringed pale buff and tips broadly white (fringes and tips less distinct on outer primaries); tail feathers pale brown, darker adjacent to shaft, shaft pale rufous-brown; side of neck, throat and underparts white (almost pure white), faint buff wash on flanks, vent and undertail-coverts; in worn plumage (from spring onwards) dark feather fringes of upperparts abraded, pale streaks on crown and nape better defined, and looks slightly less rufous above; iris brown; upper mandible dark brown to blackish, cutting edges paler, lower mandible pale brown to pinkish or whitish, tipped dark brown; legs pale brown to dark brown. Distinguished from very similar <em>C. brachydactyla</em> , often with great difficulty, mainly by more distinct supercilium, especially in front of eye, cleaner and paler underparts, warmer, paler and more spotted or scalloped (less streaked) upperparts, and slightly shorter bill, but especially by voice. Sexes similar. Juvenile is as adult, but has larger pale feather centres above, thus looking more spotted (less streaked), dull whitish underparts indistinctly and finely spotted darker, especially on breast and flanks, pale buff wash on belly and undertail-coverts. Races vary mainly in colour tones: britannica is darker and more rufous above and less white below than nominate, with stronger rufous to buff wash from flank and belly to undertail-coverts; macrodactyla is slightly paler above (less extensive dark fringes, broader whitish feather centres), on average less rufous (especially rump), than previous, purer white on breast, reduced buff wash on lower underparts, birds from Vosges (France) almost lack white on upperparts and have greatest extent of buff/rufous wash below; <em>corsa</em> resembles last, but upperparts more sharply streaked white, outer primaries fringed brighter rufous-buff, entire underparts tinged buff (less dusky on breast and belly) and, when plumage fresh, has more prominent (although still faint) brownish feather fringes on lower breast, belly and flanks than other races; <em>daurica</em> is poorly marked, resembles nominate but upperparts paler and greyer, with more extensive whitish feather centres (almost whole feather), including on rump and uppertail-coverts, which therefore contrast less with mantle; japonica resembles previous, but has upperparts darker and distinctly more rufous, with white spotting smaller (very like nominate); caucasica resembles nominate, but slightly duller and darker, less rufous, above, with whitish streaks slightly better defined; persica resembles nominate but has longer bill; tianschanica is distinctively longer-billed than nominate, also has upperparts slightly paler and more rufous, fringes of primaries slightly richer rufous-buff, lower flanks and undertail-coverts darker, washed dull pale buff; <em>bianchii</em> is close to nominate but darker and duller above (feather centres pale grey or grey-buff, not whitish), rump slightly more orange-buff with paler and more whitish centres, belly, flanks and undertail-coverts duller, washed dull buff.
Systematics History
Editor's Note: This article requires further editing work to merge existing content into the appropriate Subspecies sections. Please bear with us while this update takes place.
Formerly treated as conspecific with C. hodgsoni and C. americana, but both of these differ genetically and vocally from present species. Corsican race (corsa) moderately distinctive in morphology and voice, but a recent phylogeographic study found that corsa and caucasica are sister taxa representative of a once-widespread geographical lineage that failed to persist outside Corsica and the Caucasus (1). Geographical variation otherwise minor, to some extent clinal, plumage becoming paler and greyer and white streaking better defined E from N Europe (slightly paler, greyer birds from E European Russia and W Siberia once separated as race “rossica”), but this cline reversed in SE Russia (Amurland), NE China and Japan; also, a cline of increasing colour saturation in Europe from N (nominate race) to S & W (macrodactyla), British birds (britannica) representing W extension of cline; nominate race intergrades with macrodactyla in W (Germany/Poland region) and with daurica in E (Yenisey Basin, in SC Siberia). S races persica and tianschanica closely resemble nominate, and perhaps better merged with it. In extreme E of species’ range, proposed races kurilensis (described from Kuril Is) and orientalis (from R Sidemi, in S Ussuriland) treated as synonyms of daurica; kurilensis, however, possibly exhibits some constant differences in coloration from last, and may merit recognition; further study required. Ten subspecies recognized.Subspecies
Certhia familiaris britannica Scientific name definitions
Distribution
Certhia familiaris britannica Ridgway, 1882
Definitions
- CERTHIA
- certhia
- familiare / familiaris
- britanica / britanniae / britannica / britannicus / brittanica
The Key to Scientific Names
Legend Overview
Certhia familiaris macrodactyla Scientific name definitions
Distribution
Certhia familiaris macrodactyla Brehm, 1831
Definitions
- CERTHIA
- certhia
- familiare / familiaris
- macrodactyla / macrodactylum / macrodactylus
The Key to Scientific Names
Legend Overview
Certhia familiaris corsa Scientific name definitions
Distribution
Certhia familiaris corsa Hartert, 1905
Definitions
- CERTHIA
- certhia
- familiare / familiaris
- corsa
The Key to Scientific Names
Legend Overview
Certhia familiaris familiaris Scientific name definitions
Distribution
Certhia familiaris familiaris Linnaeus, 1758
Definitions
- CERTHIA
- certhia
- familiare / familiaris
The Key to Scientific Names
Legend Overview
Certhia familiaris daurica Scientific name definitions
Distribution
Certhia familiaris daurica Domaniewski, 1922
Definitions
- CERTHIA
- certhia
- familiare / familiaris
- daurica / dauricus
The Key to Scientific Names
Legend Overview
Certhia familiaris orientalis Scientific name definitions
Distribution
Certhia familiaris orientalis Domaniewski, 1922
Definitions
- CERTHIA
- certhia
- familiare / familiaris
- orientale / orientalis
The Key to Scientific Names
Legend Overview
Certhia familiaris japonica Scientific name definitions
Distribution
Certhia familiaris japonica Hartert, 1897
Definitions
- CERTHIA
- certhia
- familiare / familiaris
- japanensis / japanicus / japensis / japonensis / japonica / japonicum / japonicus
The Key to Scientific Names
Legend Overview
Certhia familiaris persica Scientific name definitions
Distribution
Certhia familiaris persica Zarudny & Loudon, 1905
Definitions
- CERTHIA
- certhia
- familiare / familiaris
- persica
The Key to Scientific Names
Legend Overview
Certhia familiaris tianschanica Scientific name definitions
Distribution
Certhia familiaris tianschanica Hartert, 1905
Definitions
- CERTHIA
- certhia
- familiare / familiaris
- tianschanica / tianschanicus / tianshanica / tianshanicus
The Key to Scientific Names
Legend Overview
Certhia familiaris bianchii Scientific name definitions
Distribution
Certhia familiaris bianchii Hartert, 1905
Definitions
- CERTHIA
- certhia
- familiare / familiaris
- bianchii
The Key to Scientific Names
Legend Overview
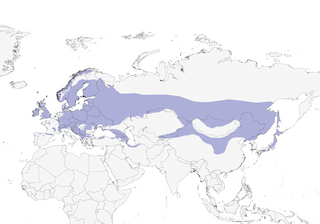
Distribution
Editor's Note: Additional distribution information for this taxon can be found in the 'Subspecies' article above. In the future we will develop a range-wide distribution article.
Habitat
Forest and woodland, generally requiring well-grown trees with many cracks and crevices in bark for foraging, roosting and nesting. Tends to favour older stands of spruce (Picea), but habitat preferences complex and apparently affected by presence or absence of C. brachydactyla. In W & C Europe, especially where range overlaps that of latter, tends to be found in beech (Fagus sylvatica) and in coniferous woodland of spruce, also fir (Abies) and to lesser extent pine (Pinus), usually at higher altitudes, but also frequents mixed and deciduous woodland, including riverine woodland, at least locally. In British Is (where C. brachydactyla absent) found in deciduous broadleaf and mixed woodland, less often coniferous forest , also in well-wooded farmland, parkland, orchards and occasionally large gardens, even in urban areas. Similarly, in European Russia (where congener also absent), strong preference for mature broadleaf and mixed forest, rather than conifers; in Caucasus found in mature broadleaf forest and alder (Alnus) forests in marshy localities, and in Iran exclusively in broadleaf, but in Siberia found in coniferous forests of larch (Larix), pine, fir and spruce, as well as mixed and riverine broadleaf woodland in lowlands. Likewise, found in mixed and coniferous woodland in Japan. In all areas, will disperse into almost any wooded habitat outside breeding season. In N of range breeds down to sea-level, but largely montane in S; in Pyrenees occurs above 1370 m, in Appennines of Italy (Abruzzo province) above 1160 m, breeds to 2230 m in Switzerland, 1800 m (occasionally 2000 m) in Austria, 1300 m in Hungary, 300–1800 m in Corsica , c. 800–1800 m in Greece, from near sea-level to 2400 m in Turkey, to c. 3000 m in Iran and Caucasus, 1500 m in Altai, to tree-line on Sayan Mts and to at least 2440 m in Tien Shan in C Asia. In E, occurs from near sea-level up to 915 m on Hokkaido, but farther S in Japan at 1065–2135 m (or to tree-line, exceptionally down to 730 m); in NE China also montane (e.g. occurs c. 400–2100 m in Changbai Shan, in Jilin), and only rare winter visitor to coastal plains.
Movement
W & S populations largely resident (e.g. most British individuals move less than 20 km), but those in more N areas tend to move S after breeding, although numbers and distances vary in irregular pattern; possibly only summer visitor in far N of range. Dispersal mid-Sept to mid-Nov (from mid-Aug in “irruption” years), with return movements mid-Mar to early May; local resident populations tend to mask presence of such migrants, but wanderers of N nominate race have reached e.g. Britain, Netherlands, Denmark, W Germany, SE Russia (including Saratov), Caspian Sea and Sea of Azov. Apparently reluctant to undertake water crossings, however, and little evidence of immigration in Britain apart from few records of vagrant nominate race; vagrants recorded Balearic Is (Mallorca), Channel Is and Faeroe Is. Also some post-breeding dispersal in N direction, and has reached Russian Lapland and White Sea. In E of range, recorded in winter in outliers of Greater Khingan Range in Mongolia, and an uncommon passage migrant and winter visitor in Korea; in NE China away from breeding areas, a few occur at Beidaihe (coastal Hebei) in late autumn and recorded Feb–Mar in Qingdao (Shandong). In addition, upland populations may undertake some altitudinal movements, e.g. in Japan, Tien Shan, W Ukraine, Caucasus and Hungary.
Diet and Foraging
Food insects , spiders (Araneae) and, in winter, also some seeds (especially pine and spruce). Forages mouse-like on trunks and larger boughs of trees, sometimes among outer foliage, using fine bill to extract prey from cracks and crevices. Usual method of progression is to fly to bottom of a tree, work upwards in jerky hops, spiralling around trunk, and then fly to bottom of another tree and repeat the process. Occasionally forages on walls, buildings, fences and bare grassy ground or among fallen conifer needles, and may flycatch. Typically found singly in winter, sometimes in pairs (pair-bond apparently sometimes persists outside breeding season, or in some regions pairs may be established in autumn), and very often joining mixed-species foraging flocks.
Sounds and Vocal Behavior
Commonest call shrill, high-pitched, but characteristically emphatic and penetrating “srrih” or “tsree”, with perceptible rolling or vibrato quality, often in regular slow series e.g. “tsree-tsree-tsree-tsree”, usually used for contact, may also be given in aggressive encounters or by begging female; also pure, plain “tee”, sometimes repeated in slow series; in heightened excitement slightly higher-pitched, thin, sharp and almost hissing “tsee” or “ziih”, slightly falling in pitch, sometimes in fast series when challenging intruding treecreeper or in slower series in alarm; flight call a short, thin, high-pitched “si” or “sit”. Song , by male only, throughout year (especially in late winter and spring), a thin, high-pitched silvery cadence up to 3 seconds in duration, falling progressively in pitch, at first thin and tremulous, gains in power and volume and terminates with sibilant flourish, comprises a block of 2–3 “srrih” notes, followed by 3–7 thin and tremulous twittering notes falling in pitch in descending trill, and, after characteristic “hiccup”, 5–14 notes (starting at higher pitch than end of second block) in fine descending ripple, the terminal note a clear falling and then rising whistle (“suih”), thus “tsee-tsee-tsi-tsi-si-si-si-si-sisisisisi-tsee”. Some geographical variation in song, possibly thinner and more delicate in Scandinavia; in Corsica (race corsa) shorter, often lacking final ripple. Subsong faint, a halting repetition of 5–6 notes, lacking plaintiveness and “ee” sound of true song. Song of present species more stereotyped and usually twice as long as that of C. brachydactyla, with three times as many notes and wider range of pitch, also less far-carrying; “mixed” singers, which imitate song of congener or, more frequently, mix part of latter’s song into own song, occur regularly in areas of range overlap.
Breeding
Season late Mar to Jun in W Palearctic and May–Jul in Japan; c. 20% of pairs double-brooded, especially in S & W of range. Monogamous; serial polygyny occasionally recorded, male mating with a second female and feeding her young, rather than, or in addition to, young of primary female. Breeding pair occupies home range, and area around nest apparently defended as a nesting territory by male, usually with song (mixed singers defend against both conspecifics and C. brachydactyla). Nest base built by both sexes, over period of generally 4–9 days (up to 20 days recorded), from conifer needles, bark fibres, grass, moss, lichen, wood chips and the like, lining added by female alone, consists of feathers, hair, wool, lichen, spider webs, eggs and cocoons etc., in conifer woods usually also moss; placed up to 16 m above ground behind flap of loose bark or in crevice on tree trunk, sometimes on or in building or stone wall, occasionally hidden among or behind vegetation, especially ivy (Hedera), or in mass of dead leaves accumulated in tree, very rarely on ground; specially designed nestboxes or artificial nest-flaps also used, especially in coniferous woodland. Clutch 2–9 (usually 5–6) eggs in W Palearctic, 3–9 (usually 3–5) in Japan, white, very finely marked with pink to reddish-brown speckles, usually as ring or cap around broader end, average size (race britannica) 15·5 × 12·1 mm; incubation by female, period 12–20 days normally (13–17 days); chicks fed by both sexes , brooded only by female, nestling period 12–19 days, mostly 15–17 days (but up to 23 days if fed by female alone); young make first attempts to feed themselves c. 7 days after fledging; temporal overlap in broods not unusual, male building new nest and female laying second clutch while still feeding first brood; family-members stay together for c. 14 days (although dependent on occurrence of second clutches, and young sometimes divided between the two parents), independent juveniles then joining mixed flocks.
Conservation Status
Not globally threatened. Fairly common in general, but unobtrusive; uncommon to rare in many areas towards N & S extremes. Densities much lower in N of range than in S, e.g. 0·04–0·5 pairs/10 ha in Finland compared with 0·25–3·43 pairs/10 ha in Germany and 1·6–8 pairs/10 ha in Corsica. European populations either stable or exhibiting declines as a result of habitat fragmentation and loss of older-growth woodland. Susceptible to effects of hard winters, especially extended periods of glazed frost or freezing rain; N & E populations tend to fluctuate in irregular pattern, possibly tied to variations in crop of spruce seeds.
- Year-round
- Migration
- Breeding
- Non-Breeding





























