Common Rosefinch Carpodacus erythrinus Scientific name definitions
Text last updated October 11, 2016
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Arabic | حسون وردي شائع |
| Armenian | Սովորական ոսպնուկ |
| Asturian | Picaflor carmñn |
| Azerbaijani | Adi mərcimək quşu |
| Basque | Gailupa karmina |
| Bulgarian | Червена чинка |
| Catalan | pinsà carminat |
| Chinese | 普通朱雀 |
| Chinese (Hong Kong SAR China) | 普通朱雀 |
| Chinese (SIM) | 普通朱雀 |
| Croatian | rujnica |
| Czech | hýl rudý |
| Danish | Karmindompap |
| Dutch | Roodmus |
| English | Common Rosefinch |
| English (United States) | Common Rosefinch |
| Faroese | Roðaígða |
| Finnish | punavarpunen |
| French | Roselin cramoisi |
| French (France) | Roselin cramoisi |
| Galician | Pimpín de peito carmín |
| German | Karmingimpel |
| Greek | (Κοινή) Ροδόσπιζα |
| Hebrew | ורדית אירופית |
| Hungarian | Karmazsinpirók |
| Icelandic | Rósafinka |
| Italian | Ciuffolotto scarlatto |
| Japanese | アカマシコ |
| Korean | 붉은양진이 |
| Latvian | Mazais svilpis |
| Lithuanian | Raudongalvė sniegena |
| Malayalam | റോസ്കുരുവി |
| Marathi | गोरली |
| Mongolian | Улаавар бужмар |
| Norwegian | rosenfink |
| Persian | سهره گلی |
| Polish | dziwonia |
| Portuguese (Portugal) | Peito-carmim |
| Romanian | Mugurar roșu |
| Russian | Обыкновенная чечевица |
| Serbian | Rumenka |
| Slovak | červenák karmínový |
| Slovenian | Škrlatec |
| Spanish | Camachuelo Carminoso |
| Spanish (Spain) | Camachuelo carminoso |
| Swedish | rosenfink |
| Thai | นกจาบปีกอ่อนสีกุหลาบ |
| Turkish | Çütre |
| Ukrainian | Чечевиця євразійська |
Carpodacus erythrinus (Pallas, 1770)
Definitions
- CARPODACUS
- erythrinus
The Key to Scientific Names
Legend Overview
Field Identification
13–15 cm; 19–33 g. Medium-large rosefinch with stubby or bulbous bill and notched tail. Male nominate race breeding has forehead to nape bright scarlet (variable in extent, and often duller or with browner bases showing through); lores dusky grey-brown, sometimes continuing behind eye as brownish eyestripe, cheek and ear-coverts brown or reddish-brown, washed brighter red; upperparts brown, edged paler, and washed bright red or reddish-pink (duller or browner in worn plumage), rump variably pinkish to deep red (depending on age) or with brownish tips, uppertail-coverts brown, washed red; tail brown, feathers broadly edged paler or reddish-brown; upperwing dark brown, median coverts finely edged paler and tipped pale pink or pinkish-buff, tips of greater coverts more uniform brownish-pink and rarely paler (both sets forming wingbars), flight-feathers finely edged pale buff or buffish-brown and tinged pink to light reddish towards tips, tertials more broadly edged pale pink or pinkish-buff; chin and throat to upper breast variable, bright red or duller, and merging with red on face and side of throat (may show dark malar stripe); side of breast and belly buffish-white or washed reddish, flanks slightly browner and streaked or washed reddish, undertail-coverts whitish; iris dark brown or black; bill grey to dark grey-brown with yellowish-brown to pinkish base of lower mandible, or plain dull olive; legs brown or pinkish-brown. Non-breeding male in fresh plumage (winter) has crown dull red to reddish-pink, feathers sometimes with brown bases, and upperparts (including rump) greyer, tips of wing-coverts warm buffish-brown, brightest pink to reddish-orange on side of throat to centre of breast. Female lacks bright red, has head and upperparts light olive-brown, tinged grey, with fine blackish streaks on forehead and crown, face slightly paler, may have short whitish-buff supercilium and subocular crescent, and broad pale buff moustachial stripe bordered by dark brown malar stripe; tail dark brown, finely edged light olive-brown; upperwing dark brown, median and greater coverts edged pale brown and tipped pale buff (forming double wingbar), flight feathers-edged pale olive-brown, more buffish-white on primaries; underparts buffish-white, tinged olive on breast and flanks, and streaked finely darker, belly to undertail-coverts whitish; bare parts much as for male. Juvenile is like female, but tinged buff-brown on head and face and finely streaked darker on forehead and crown, broadly streaked across upperparts, tips of median and greater coverts and edges of flight-feathers buff to warm buff-brown, tertials more broadly edged and tipped pale buff or whitish, underparts buffish and more heavily streaked, bill greyer; first-summer and second-summer male like adult female or slightly browner on head and upperparts (sometimes breeds in this plumage), or may have variable amounts of red on forehead, face and ear-coverts, also reddish feather tips on rump and underparts; adult-like plumage acquired in late summer of second year, forehead to crown deep red, nape and upperparts also tinged red, rump orange, becoming reddish-orange on lower rump, uppertail-coverts brown, tips of upperwing-coverts broadly pinkish-buff, chin to breast as on adult male or variably tinged or mottled with pink, white, yellow or pale orange, side of breast and flanks warm buff-brown and belly pale pink. Races differ mainly in intensity and extent of red on head, upperparts and underparts, but differences obscured in worn plumage and outside breeding season: grebnitskii is darker carmine-red than nominate, male has deeper red upperparts overlying dark grey-brown, paler or more pinkish-red underparts (extending to belly and flanks) than roseatus, female darker or browner above and below; kubanensis male is similar to nominate, but head and breast deeper red, mantle and scapulars brown, tinged reddish, and pinkish-red on underparts extends to upper belly and flanks, female paler and greyer, less olive-tinged, than nominate; ferghanensis male has head and upperparts darker brown than previous, less reddish suffusion on upperparts, below is more extensively rose-red on belly and flanks, female also darker or browner; <em>roseatus</em> breeding male is deeper red than males of other races, deep or rich carmine on head and upperparts , mantle and scapulars streaked blackish, rump bright carmine-red, red extending down to breast and lightly onto belly and flanks, female darker brown and more heavily streaked on crown, upperparts and underparts.
Systematics History
Editor's Note: This article requires further editing work to merge existing content into the appropriate Subspecies sections. Please bear with us while this update takes place.
Sometimes isolated in Erythrina. Geographical variation weak and, except for race roseatus, not well defined; races not always separable in worn plumage. Previously published phylogeographical analysis of mtDNA sequences, however, suggested existence of three recently diverged groups, corresponding to, respectively, Caucasus, CW Eurasia and NE Eurasia; recent re-evaluation of mtDNA data using coalescence methods, and addition of sequence data from a sex-linked gene, supported distinctiveness of Caucasian group but not the two other groups; overall, coalescence methods indicated lack of gene flow among the three groups and population expansion in CW Eurasian and NE Eurasian groups, the three groups corresponding to described races, further supporting their validity (1). Five subspecies recognized.Subspecies
Carpodacus erythrinus erythrinus Scientific name definitions
Distribution
Carpodacus erythrinus erythrinus (Pallas, 1770)
Definitions
- CARPODACUS
- erythrinus
The Key to Scientific Names
Legend Overview
Carpodacus erythrinus grebnitskii Scientific name definitions
Distribution
Carpodacus erythrinus grebnitskii Stejneger, 1885
Definitions
- CARPODACUS
- erythrinus
- grebnitskii / grebnitzkii
The Key to Scientific Names
Legend Overview
Carpodacus erythrinus kubanensis Scientific name definitions
Distribution
Carpodacus erythrinus kubanensis Laubmann, 1915
Definitions
- CARPODACUS
- erythrinus
- kubanensis
The Key to Scientific Names
Legend Overview
Carpodacus erythrinus ferghanensis Scientific name definitions
Distribution
Carpodacus erythrinus ferghanensis (Kozlova, 1939)
Definitions
- CARPODACUS
- erythrinus
- ferghanensis
The Key to Scientific Names
Legend Overview
Carpodacus erythrinus roseatus Scientific name definitions
Distribution
Carpodacus erythrinus roseatus (Blyth, 1842)
Definitions
- CARPODACUS
- erythrinus
- roseata / roseatus
The Key to Scientific Names
Legend Overview
Distribution
Editor's Note: Additional distribution information for this taxon can be found in the 'Subspecies' article above. In the future we will develop a range-wide distribution article.
Habitat
Lowland to montane moist forests, woodlands and thickets of willows (Salix), alder (Alnus), poplar (Populus), tamarisks (Tamarix), scrub and bushes in taiga forest edges and clearings, also riverine thickets, reedbeds and patches of bushes in meadows, forest edges, hedges, orchards, cherry (Prunus) trees and edges of cultivation; in forest-steppe and higher areas of montane foothills occurs in bracken (Pteridium), dwarf willows, juniper (Juniperus) and on bush-covered slopes with isolated birch (Betula) and firs (Abies); breeds even in some city centres, e.g. Ulaanbaatar (Mongolia) and Almaty (Kazakhstan). In Europe breeds mostly below 200 m, but to above 1850 in some parts of range; in Kazakhstan to 2800 m, and in C Asia at 2500–3700 m; in W Himalayas race ferghanensis breeds at 2300–3600 m, lower, to 2000 m, in E Afghanistan and to 3900 m (occasionally to 4200 m) in Ladakh; farther E, roseatus breeds at 2000–4550 m in montane and submontane junipers along and above tree-line, also in thornbushes on plains, alpine slopes and hillsides to edges of terraced cultivation. In non-breeding season occurs at lower levels (usually below 1500–2000 m) in similar habitat in open areas of foothills, plains, reedbeds and edges of cultivation, principally paddyfields, mustard and sugar cane; on passage occurs in similar habitat, also in coastal woodlands and scrub.
Movement
Migratory and partially migratory. Most movements nocturnal, on spring passage also diurnal, alone, in small flocks (often comprising same-age birds) or up to 200 together, occasionally flocks of several hundred birds. Nominate race entirely migratory, in non-breeding season moves between SE and E from late Jul to early Nov to wintering areas from S Nepal and N India E to N Myanmar and NW Thailand, adults leaving ahead of juveniles. Those from C & E Europe head E to areas N of Caspian Sea before continuing S to SE (joining with birds from W Russia and N Kazakhstan) across C Asian deserts; individuals ringed in Scandinavia and NW Europe take route from Norway and Finland through Uzbekistan, and from Kaliningrad (W Russia) to E Turkmenistan; passage S through Altai and NE & E Kazakhstan mostly between mid-Jul and mid-Sept presumably of Siberian breeders; in Tien Shan and Pamir-Alai Mts populations of nominate and race ferghanensis converge, and one of the commonest migrants in Aug and early Sept, with high concentrations in valleys, also farther S in Tajikistan large numbers in town parks, gardens and around settlements; also recorded off-passage for up to 7–10 days N of Tien Shan in SE Kazakhstan, before crossing mountain ranges into S Asia, passage mostly over by mid-Sept, and thereafter only small numbers to early Nov; passage of kubanensis from E Turkey and Caucasus from end Aug to end Sept/early Oct (single mid-winter record S Turkey), mostly E through N Iran and Afghanistan; locally scarce or rare on passage (mostly singles) E Israel mid-Aug to mid-Oct (more numerous than in winter and on spring passage) and Sinai (NE Egypt), uncommon passage migrant United Arab Emirates late Aug to early Nov, also scattered records in winter to early Mar. Most migrants (of all three races) arrive N Pakistan between Aug and Oct, scarce or absent S Pakistan in autumn but more numerous in spring. Race grebnitskii winters from SC China and N Thailand E to N Vietnam; departs from breeding areas later than in W of range, passage through NE Russia and Mongolia from late Aug and early Sept, rare on passage Ussuriland and Japan, and presumably moves through N & C China, with passage recorded NE China mostly during Sept, regular passage migrant mid-Aug to end Oct in Korea; scarce but regular Hong Kong in winter from end Oct, but most present between late Dec and early Mar. S breeding populations of races ferghanensis, kubanensis and roseatus are altitudinal or short-distance migrants, moving locally to foothills and valleys in lowlands of N & C India and E to N Indochina, kubanensis possibly also a scarce winter visitor in Nepal; in Bhutan movement of roseatus and nominate in Oct–Nov to lower levels, and latter present in small numbers to at least Jan. Returns to breeding areas from late Apr to early Jun, passage through Pakistan, N India and Bhutan mostly Apr to mid-May and first birds in Himalayas and C Asian mountains from mid-Apr; arrives at higher altitudes in late May and Jun, first returns in Caucasus from late Apr and in C Turkey and N Iraq in early May. Passage through Tajikistan from mid-Apr to mid-May, through S Urals mostly during May, and reaches S Finland and St Petersburg area from E or SSE in mid-May, slightly later on Baltic coast of Estonia and at end of May in C Sweden; at W end of European breeding range arrives Austria late May and early Jun; in C Siberia (Tomsk region) and NE Altai passage mid-May to early Jun, and some of grebnitskii breeding farthest N arrive on territory in Russian Far East early to middle Jun. Three males tagged with geolocators at their breeding grounds in S Sweden wintered in Pakistan and W India and showed loop-migration, with more northerly routes in autumn than in spring; during autumn migration the birds used several staging sites in Central Asia for prolonged periods, while during spring migration they made a few longer stops while still in N India or Central Asia, before migrating at fast speeds towards the breeding grounds; during the autumn migration the birds left the breeding sites in late Jul–early Aug and arrived to the Indian subcontinent in Oct, while the spring migration begun in mid-Apr and arrival at the breeding grounds was in the second half of May (2). On passage occurs regularly (mostly dispersing first-year individuals) in Europe W to Switzerland, British Is and N to Iceland and C Norway, also S to United Arab Emirates, and has wintered in Oman; in E of range occurs on passage in Japan, Commander Is and, less frequently (and mostly in spring), in Aleutian Is and Pribilof Is and W Alaska. Vagrant N to Faeroes and W to Canary Is (Lanzarote), Portugal, islands in Mediterranean and Morocco.
Diet and Foraging
Mostly plant and tree seeds , buds, catkins, shoots, leaves, fruit and berries , also nectar; also insects and larvae and other arthropods. Seeds and berries include those of juniper, larch (Larix), spruce (Picea), pine (Pinus), willow, aspen and other poplars, birch, alder, oak (Quercus), elm (Ulmus), maple (Acer), lime (Tilia), lilac (Syringa), mulberry (Morus), hemp (Cannabis), knotgrass (Polygonum), dock (Rumex), buckwheat (Fagopyrum), chickweed (Stellaria), pearlwort (Sagina), campion (Silene), buttercup (Ranunculus), radish (Raphanus), currant (Ribes), bramble (Rubus), apple (Malus), pear (Pyrus), cherry, rowan (Sorbus), meadowsweet (Filipendula), vetchling (Lathyrus), crane's-bill (Geranium), buckthorn (Rhamnus), alder buckthorn (Frangula), spurge-laurel (Daphne), willowherb (Epilobium), bilberry (Vaccinium), cow parsley (Anthriscus), comfrey (Symphytum), speedwell (Veronica), plantain (Plantago), dandelion (Taraxacum), elder (Sambucus), guelder-rose (Viburnum), raspberry (Rubus), snowberry (Symphoricarpos), sow-thistle (Sonchus), honeysuckle (Lonicera), figs (Ficus), Lantana, Maesia, Trema, wood-rush (Luzula), sedges (Carex), grasses and cereals (Gramineae), also bamboo and millet. Sips nectar of Erythrina, Bombax, Butea, Woodfordia and from other blossoms (forehead and throat feathers often become stained yellowish). Arthropods taken (mostly in summer) include adult and larval small dragonflies and damselflies (Odonata), bugs (Hemiptera) including aphids (Aphidoidea), moths (Lepidoptera) caddis flies (Trichoptera), flies (Diptera), beetles (Coleoptera), spiders (Araneae) and mites (Acari). Nestlings fed with pulp of regurgitated seeds and insects. Forages on the ground , in grasses and bushes or low herb vegetation; also perches and forages at all levels in trees when feeding on buds or fruit; hops on ground. Removes outer scales from buds, fruit, cereals or flowerheads and eats soft central core; nibbles fresh leaves and conifer needles. Consumes up to 15 buds in 5–10 minutes. Often at edges of roadside and saltpans, taking salt minerals; also takes mortar from walls and urine-soaked earth from around horse stables. Forages singly, in pairs and in small groups; forms larger post-breeding flocks of several family groups, in non-breeding season also larger flocks of 100–200 individuals, and joins mixed-species flocks with other seed-eaters, including other finches, sparrows (Passer) and buntings (Emberizidae).
Sounds and Vocal Behavior
Song a regularly and monotonously repeated (in breeding season), slowly rising whistled "weeja-wu-weeeja", "weeech-wu-weecha" or variations, including "tway-tyu-tu-u-ooh", "tiu-wee-tiu", "tsitsewitsa" and "te-te-wee-chew"; in small, isolated local populations, all males sing same song type ("song neighbourhood"); generally silent outside breeding season, but male begins singing in early spring before departure from wintering areas. Call a distinctive, clear and rising "oooeet", "too-ee", "uueet", "dooei" or "djay-ee", or an interrogative "tway"; in flight or when about to fly gives short "zik" or "zlit"; anxiety or alarm note a sharp or harsh "chay-eeee", similar to a note of Chloris chloris.
Breeding
Season May–Aug; one brood. Monogamous; occasionally polygamous in areas with apparent excess of immature (brown-plumaged) males; pair-bond endures for single season, exceptionally longer, in one case for up to four successive years (with temporary new partner in third year, before re-pairing with previous partner). Young males sometimes co-operative helpers, assisting in feeding of young. Solitary or loosely colonial. Territory used for courtship and nesting, but most food-gathering done outside (male forages up to 3 km from territory), defended by both sexes, mostly by male, defence gradually declining during fledging period; size of territory variable, c. 1500 m2 in Finland, 1600 m2 in Urals, 3000 m2 in Poland and 1600–3000 m2 in S Sweden, exceptionally to 11,050 m2. Males arrive in breeding area (often in small flocks) ahead of females, and pairing follows arrival of latter; male sings from regular songposts early in breeding season, and may attract female and subsequently move to different area to breed. In display, partners close together on ground, rock or branch, male adopts posture with head held high, crown feathers raised, wings drooped and slightly quivering, and tail partly raised, and slowly circles female, partners may also take turns in circling each other with head-up posture; male also stands in front of female and swings body slowly from side to side while vibrating drooped wings, he also bows towards female and then throws back head and gives rapid burst of song; other courtship includes bill-touching or head-pecking, mate-guarding (while female feeding) and slow butterfly-like flight with stiff wingbeats by male, also courtship-feeding by male, usually later in season during incubation and brooding of young. Nest built by female, a loose or untidy cup of twigs, plant stems and fibres, grass, flowerheads, plant down, moss, lichens and animal hair, placed low down (usually 1–2 m from ground, exceptionally up to 10 m) in bush, juniper or spruce or willow sapling, well hidden in tangle of foliage or against trunk, occasionally in scrub tangle, rarely on ground. Clutch 4–6 eggs, pale bluish-green with violet-grey blotches and sparse blackish-brown or purplish spots and streaks; incubation by female alone, period 11–14 days; chicks fed and cared for by both parents, nestling period 10–13 days; young leave nest before able to fly, fed by parents for 2 weeks after leaving nest. Breeding success: of 620 eggs in 273 nests in S Finland, 62% hatched and 54% of chicks fledged, average of 3 young per nest or 4·6 per successful nest, most losses due to predation by weasels (Mustelidae) and crows (Corvidae), in same study females deserted easily and presence of Red-backed Shrike (Lanius collurio) in nesting area likely to cause desertion; of 246 eggs in 49 clutches in four-year study in Sweden, 67·5% hatched and 60·6% of chicks fledged, average 3 young per nest, most losses due to mustelids, also domestic cats (Felis catus); in St Petersburg area (NW Russia), Mecklenburg (NE Germany) and NW Kazakhstan, success rates variable from 2·3 young per successful nest (of 12 nests) to 3·4 young per successful nest (of 214 eggs), and in Kazakhstan study high failure rate of 60–70% due mainly to crows, raptors, mustelids and human interference. Many breed in first year, usually when in immature plumage; breeding by first-years largely dependent on number of older males, sex ratio in population, density and availability of nest-sites. Longevity at least 9 years.
Conservation Status
Not globally threatened (Least Concern). Common to locally common. Estimated European breeding population between 505,000 and 631,000 pairs, most in Finland and Belarus; up to 50,000 pairs in Turkey and up to 10,000,000 pairs in Russia. Densities of 200 pairs/km² in pine-birch forest in C Siberia, 270 pairs/km² in Kyrgyzstan and 300 pairs/km² in water meadows in Estonia. In N Europe numbers increased since mid-1940s in Fennoscandia and NW Russia, now breeds along White Sea coast and in Murmansk area. In C Europe range expanded since 1930s W to NW Germany, Netherlands and Britain and in S to Slovenia and Austria; increase largely involved first-year birds arriving in autumn (linked to increase in numbers in Scandinavia), with a corresponding increase in numbers returning along same route in spring; first bred in Denmark 1972 and in Austria in 1973, colonized coastal belt of E Germany from 1968, and first bred in W Germany and in Britain in 1982 (then at up to five locations in next ten years); in 1992 unprecedented influx of more than 90 into Britain between mid-May and Jun resulted in widespread breeding by at least eight pairs, but numbers subsequently declined to only occasional breeding. First bred in France in 1985 (increasing to at least 30 pairs by 1993) and Netherlands in 1987 (c. 30–60 pairs by 1994, but only one in 2005); in Switzerland breeding first attempted in 1983, and successfully in 1989, and ten pairs by 1994; Belgium held nine territories in 1994. In E Asia, thought to have bred in N highlands of Korea in 1950s and 1960s, when many breeding-season records, and also several earlier such records; insufficient information on situation in more recent years.
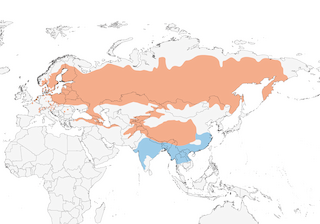
- Year-round
- Migration
- Breeding
- Non-Breeding


































