Eurasian Blue Tit Cyanistes caeruleus Scientific name definitions
Text last updated January 30, 2013
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Albanian | Trishtili i kaltër |
| Arabic | قرقف أزرق |
| Armenian | Երկնագույն երաշտահավ |
| Asturian | Beranñn ferreru |
| Azerbaijani | Adi arıquşu |
| Basque | Amilotx urdina |
| Bulgarian | Син синигер |
| Catalan | mallerenga blava eurasiàtica |
| Croatian | plavetna sjenica |
| Czech | sýkora modřinka |
| Danish | Blåmejse |
| Dutch | Pimpelmees |
| English | Eurasian Blue Tit |
| English (United States) | Eurasian Blue Tit |
| Faroese | Blátíta |
| Finnish | sinitiainen |
| French | Mésange bleue |
| French (France) | Mésange bleue |
| Galician | Ferreiriño azul |
| German | Blaumeise |
| Greek | (Ευρωπαϊκή) Γαλαζοπαπαδίτσα |
| Hebrew | ירגזי כחול |
| Hungarian | Kék cinege |
| Icelandic | Blámeisa |
| Italian | Cinciarella |
| Japanese | アオガラ |
| Latvian | Zilzīlīte |
| Lithuanian | Mėlynoji zylė |
| Norwegian | blåmeis |
| Persian | چرخ ریسک سرآبی |
| Polish | modraszka |
| Portuguese (Portugal) | Chapim-azul |
| Romanian | Pițigoi albastru |
| Russian | Лазоревка |
| Serbian | Plava senica |
| Slovak | sýkorka belasá |
| Slovenian | Plavček |
| Spanish | Herrerillo Común |
| Spanish (Spain) | Herrerillo común |
| Swedish | blåmes |
| Turkish | Mavi Baştankara |
| Ukrainian | Синиця блакитна |
Cyanistes caeruleus (Linnaeus, 1758)
Definitions
- CYANISTES
- caeruleus
The Key to Scientific Names
Legend Overview
Field Identification
11–12 cm; 7.5–14.7 g. Small, small-billed, compact tit with rounded head shape.Male nominate subspecies has forehead and upper lores white and merging with white supercilia, which join on center of nape; forecrown blue, becoming deeper blue on hindcrown; lores and eyestripe narrowly blackish, merging with deep blue on lower nape and side of neck; center of lower nape and upper mantle pale grayish-blue, rest of mantle, back and scapulars bluish-green, rump lighter, more yellowish-green, uppertail-coverts bright blue; tail blackish-blue, broadly fringed bright blue, outermost rectrix narrowly fringed whitish; upperwing-coverts and alula grayish, broadly fringed deep blue, greater coverts tipped white (forming narrow wingbar), flight-feathers dark gray, broadly fringed bright blue, tertials broadly tipped whitish; cheek and ear-coverts white; chin and upper throat black, merging narrowly across throat side with deep blue on lower side of neck; underparts yellow, tinged greenish-olive on breast, brighter yellow on flanks, center of breast may show narrow blackish line (often concealed), and center of belly may be whitish; undertail-coverts creamy; axillaries and underwing-coverts yellow; in worn plumage, slightly bluer on crown, flight-feathers and tail, upperparts grayer or less green, white tips of greater coverts and flight-feathers abraded or absent, bib slightly darker black, and blackish line on center of breast often more prominent; iris dark brown to blackish-brown; bill dark slate to black, paler cutting edges; legs slaty to blue or slate-gray. Female is on average slightly duller than male, but otherwise very similar. Juvenile has forehead , supercilium and center of nape dull yellow, crown and neck side darker, upperparts grayer, tail duller blue, upperwing-coverts fringed with greenish-blue, tips of greater coverts and tertials yellowish-white, flight-feathers edged grayish-blue, face and underparts dull yellow (becoming whiter with age), gray on chin and upper throat, and no dark central line on breast. Subspecies differ mainly in color tones, both above and below: <em>obscurus</em> is as nominate, but crown slightly darker blue, mantle slightly darker or greener (less bluish-gray), white tips on greater coverts and tertials slightly narrower, underparts deeper yellow with usually obvious narrow line on central breast; <em>ogliastrae</em> is similar to previous, but mantle slightly bluer, wing-coverts deeper blue, underparts bright yellow, female often as brightly colored as male; <em>balearicus</em> is as nominate, but mantle paler or grayer, underparts slightly paler, whiter on breast and belly, line on center of breast narrower; calamensis is very like nominate but slightly smaller; orientalis is as nominate, but upperparts olive-gray, tinged yellowish, and underparts brighter or paler yellow; <em>satunini</em> is also as nominate, but upperparts slightly darker, olive-gray, underparts pale yellow, flanks grayish; raddei is as last, but upperparts slightly darker olive (and less gray), underparts deep yellow; persicus has shorter and thinner bill, upperparts pale bluish-green, tinged grayish, underparts variable, from rich yellow on breast and whiter on belly and undertail-coverts to uniform whitish-yellow; <em>ultramarinus</em> resembles nominate, but crown black with glossy dark bluish tips, broad black eyestripe (narrow across lores) merging broadly on side of nape with black of hindneck, upperparts mostly dark bluish-gray (rump may be tinged greenish), tertials and secondaries broadly tipped white, lower neck side, chin and upper throat broadly black, underparts deep yellow with pronounced blackish ventral line; cyrenaicae is as last but slightly smaller, and has narrower white band on forehead, darker or duller blue mantle, slightly duller yellow underparts.
Systematics History
Editor's Note: This article requires further editing work to merge existing content into the appropriate Subspecies sections. Please bear with us while this update takes place.
Previously considered conspecific with African Blue Tit (Cyanistes teneriffae); in HBW, subspecies ultramarinus and cyrenaicae inappropriately retained in present species (1). Hybridizes with Azure Tit (Cyanistes cyanus), especially following years when latter expands west in irruptive manner (hybrids described as separate taxon, “pleskii”); also hybridizes rarely with Great Tit (Parus major). Nominate subspecies intergrades with obscurus, ogliastrae and satunini; also, satunini intergrades with persicus, and it is uncertain which subspecies breeds in northwestern Syria. Subspecies raddei described not in Parus (2) but in present genus (original checked). Nine subspecies recognized.
Subspecies
Cyanistes caeruleus obscurus Scientific name definitions
Distribution
Cyanistes caeruleus obscurus (Pra_ák, 1894)
Definitions
- CYANISTES
- caeruleus
- obscurum / obscurus
The Key to Scientific Names
Legend Overview
Cyanistes caeruleus caeruleus Scientific name definitions
Distribution
Cyanistes caeruleus caeruleus (Linnaeus, 1758)
Definitions
- CYANISTES
- caeruleus
The Key to Scientific Names
Legend Overview
Cyanistes caeruleus ogliastrae Scientific name definitions
Distribution
Cyanistes caeruleus ogliastrae (Hartert, 1905)
Definitions
- CYANISTES
- caeruleus
- ogliastrae
The Key to Scientific Names
Legend Overview
Cyanistes caeruleus balearicus Scientific name definitions
Distribution
Cyanistes caeruleus balearicus (Jordans, 1913)
Definitions
- CYANISTES
- caeruleus
- balearica / balearicus
The Key to Scientific Names
Legend Overview
Cyanistes caeruleus calamensis Scientific name definitions
Distribution
Cyanistes caeruleus calamensis (Parrot, 1908)
Definitions
- CYANISTES
- caeruleus
- calamensis
The Key to Scientific Names
Legend Overview
Cyanistes caeruleus orientalis Scientific name definitions
Distribution
Cyanistes caeruleus orientalis Zarudny & Loudon, 1905
Definitions
- CYANISTES
- caeruleus
- orientale / orientalis
The Key to Scientific Names
Legend Overview
Cyanistes caeruleus satunini Scientific name definitions
Distribution
Cyanistes caeruleus satunini Zarudny, 1908
Definitions
- CYANISTES
- caeruleus
- satunini
The Key to Scientific Names
Legend Overview
Cyanistes caeruleus raddei Scientific name definitions
Distribution
Cyanistes caeruleus raddei Zarudny, 1908
Definitions
- CYANISTES
- caeruleus
- raddei
The Key to Scientific Names
Legend Overview
Cyanistes caeruleus persicus Scientific name definitions
Distribution
Cyanistes caeruleus persicus (Blanford, 1873)
Definitions
- CYANISTES
- caeruleus
- persicus
The Key to Scientific Names
Legend Overview
Hybridization
Hybrid Records and Media Contributed to eBird
-
Eurasian Blue x Azure Tit (hybrid) Cyanistes caeruleus x cyanus
Distribution
Editor's Note: Additional distribution information for this taxon can be found in the 'Subspecies' article above. In the future we will develop a range-wide distribution article.
Habitat
In Europe, mostly lowland and submontane deciduous woodlands, principally containing oak (Quercus) and birch (Betula), and broadleaf evergreen woods, also thickets, copses, hedgerows, areas of scrub with scattered trees, edges of cultivation, orchards, parks and gardens, including suburban areas and city centres; generally avoids large stands of conifers, although occurs in mature conifers in non-breeding season when in mixed flock. In E Mediterranean, occurs in high-altitude pine (Pinus) and oak in Jordan, cedar (Cedrus) forest in NW Syria and montane oak woodlands in Iran; in more arid areas in SE parts of range occurs mainly in riverine woodland, parks and gardens. In N Africa in cork oak (Quercus suber) forest and in Atlas oak (Quercus faginea) and evergreen oak (Quercus ilex), also cedar and montane juniper (Juniperus) forests in Morocco and N Libya; farther S, resident in palm groves of Saharan oases. In non-breeding season generally found in wider range of habitats containing trees and shrubs, but also in reedbeds. Sea-level to mountains: in breeding season to 1540 m in Austrian Alps, to 1700 m in Switzerland (most breeding below 1250 m), to 1800 m in Pyrenees, to 1525 m in Greece, Turkey and NW Iran (exceptionally to 2000 m in Greece), and to at least 2040 m in SW Iran; in Caucasus breeds up to tree-line at c. 3500 m; to 1800 m or higher in Morocco.
Movement
Mainly resident in C & S of range, partially migratory in N; also some seasonal altitudinal shifts. Evidence from ringing in Britain is that most movements are of 2–28 km (95% of recoveries within 10 km of ringing site, females moving farther than males), and none beyond 100 km; birds from higher altitudes of N Scotland make post-breeding descent to lower valleys. In study over four years 2007–2010 in 1·6-km² woodland area in S Norway in which elevational gradient (from 105 m to 266 m) caused phenological delay in vegetation and in peak caterpillar abundance at higher levels, present species and Parus major made similar post-fledging shifts, moving mean distance from nest to site of observation of 134 m (range 6–1036 m); on average, families moved upslope, suggesting that they were able to track environmental phenology, although most did not move far from nest-site (possibly because parents wished to defend year-round territory and could not afford to leave for longer periods) (3). Otherwise, N European populations (juveniles and adult females) more regularly move S & W in Sept to late Oct to non-breeding grounds in NC Europe and S to around Mediterranean, arriving late Oct and present to mid-Mar in S Greece and mid-Nov to late Feb in Lebanon; rarely farther S, e.g. vagrant in NE Syria and Israel. Numbers recorded on passage at watchpoints vary annually, frequently in low thousands through passes in French Alps, and sometimes very large numbers in Baltic region; occasionally on irruption scale (when overall population at high level), as in autumn 1957, when considerable numbers moved from N & NE range into Baltic area and along S North Sea coast and many arrived on E & S coasts of Britain. Most movements involve first-years, and few adults involved. Longest distances recorded in ringing studies include movements from SE Baltic coast (Kaliningrad) to S Spain (2200 km WSW) and to Venice, in Italy (1260 km SSW), and from Germany to S Spain (1480 km SW).
Diet and Foraging
Food includes small (usually c. 1 cm) invertebrates and larvae , also some fruit and seeds. Animal items principally springtails (Collembola), grasshoppers (Orthoptera), damselflies (Odonata), earwigs (Dermaptera), bugs (Hemiptera), moths (Lepidoptera, especially of family Tortricidae), lacewings (Neuroptera), spiders (Araneae), flies (Diptera), woodlice (Isopoda), ants (Formicidae), beetles (Coleoptera), harvestmen (Opiliones), millipedes (Diplopoda), slugs and snails (Gastropoda), ticks (Acari). Fruit and seeds, taken mainly in non-breeding season, include those of pine, cypress (Chamaecyparis), spruce (Picea), yew (Taxus), ash (Fraxinus), birch, beech (Fagus), box (Buxus), sycamore and maple (Acer), chestnut (Castanea), oak, olive (Olea), mulberry (Morus), fig (Ficus), mistletoe (Viscum), blackberry (Rubus), currant (Ribes), plums and cherries (Prunus), apple (Malus), pear (Pyrus), elder (Sambucus), rose (Rosa), hawthorn (Crataegus), dogwood (Cornus), snowberry (Symphoricarpos), walnut (Juglans), honeysuckle (Lonicera), sea-buckthorn (Hippophae), privet (Ligustrum), nightshade (Solanum), knapweed (Centaurea); also takes sap of birch, sycamore, maple, poplar (Populus), walnut and vine, and nectar from flowers of willow (Salix), plum, cherry, currant, aloe (Aloe), Hibiscus and Tecomaria in spring. Details of diet determined largely by seasonal abundance of various items. Solitary, in pairs or, in autumn, in small family groups; in autumn and winter sometimes in larger flocks, e.g. in SE of range flocks containing several hundred individuals recorded; also in mixed-species flocks in non-breeding season. Partners usually remain close to breeding territory during non-breeding season and forage with other insectivorous species, including Long-tailed Tit (Aegithalos caudatus), Goldcrest (Regulus regulus) and Eurasian Treecreeper (Certhia familiaris), in roving flock, range of which (up to 10 ha) includes several breeding territories. Winter flocks gradually reduced in size towards end of winter as pairs re-establish breeding territories. Active, agile and restless. Forages at all levels in trees, bushes and shrubs, occasionally on ground but usually only very briefly. Examines leaves, buds and slim branches with great thoroughness (may spend up to 30 minutes in single tree), but in winter often moves rapidly through foliage when foraging. Frequently hangs by one leg from slender twigs or leaves; easily locates insects under loose bark (which it strips) or in leafmines or galls; gleans foliage, feeds on aphids (Aphidoidea) gathered on bushes; taps reeds to locate concealed larvae. Hovers briefly while inspecting foliage, and occasionally pursues insects in flight. Visits birdtables and feeders, where it takes household scraps, including bread, cheese, fat, peanuts and variety of seeds , especially of sunflowers (Helianthus); large seeds taken to nearby branch, where opened while held in foot or wedged in bark and hammered with rapid strikes of the bill. Well known in parts of range (e.g. Britain) for its habit of piercing milk-bottle tops on doorsteps in order to sip cream; in more extreme cases, has been known to peck at and remove fresh putty from windows.
Sounds and Vocal Behavior
Very vocal. Contact note between partners or members of flock (frequently used throughout year) a short and thin “tsee”, singly or in series; scolding or churring “churrrrit”, rising towards end, with varying emphasis, given especially in alarm or at approach of predator, may be preceded or followed by single or multiple sharp “tsee” or thin, high-pitched “seeee” or “pit” notes, e.g. “pit pit churrr” or “drrrrrrrt tit tit tit”, and frequently repeated when agitated; in N Africa also sharp calls similar to song of European birds, “tsee-tsi-brree tsee-tsi-brree” or “chi-chichiwee” and a more metallic “pichoo”. Also high-pitched “zeedle” by both sexes during courtship and copulation; female on nest also gives short, explosive hissing note in aggressive defence display to intruder or predator; nestlings and recently fledged birds high-pitched and almost incessant “tsee-tsee-tsee-tsee-tsee-tsee”. Song a clear, high-pitched and lilting “tsit-sit-sissiwit” or “tsee-tsee-tsee-tsü-tsühühühühühü” or “psi-psi-tsatsatsa, psi-psi-tsatsatsa”, frequently repeated after short pauses, also shorter variations, including “tsee-tsee see-see”, “siss-seedo” or “psi-dada, psi-dada, psi-dada”; individual males have repertoire of 3–8 songs, and song of female similar to male’s. Geographical variation not marked within Europe, but songs in Europe differ from those in NW Africa; race ultramarinus has song closer to that of Parus major, with series of disyllabic metallic “tizi” notes (songs on Corsica and Mallorca somewhat similar).
Breeding
Season Apr to late Jun, with egg-laying, clutch size and breeding success closely linked to timing of emergence and abundance of caterpillars (principally of Tortrix moths); two broods frequent in parts of Europe, but rare Britain, Germany, Corsica and Morocco. Monogamous, but polygamous males not infrequent where breeding density high; pair-bond usually lifelong. Territorial and solitary breeder; territory usually well defined by early Feb, and defended against interlopers, but pairs sometimes nest in close proximity, including case of two pairs in same nestbox; all females mated to polygamous male nest within single territory, and male feeds young in all nests. Male display includes slow moth-like flights and glides towards perched female, interspersed with intervals when crest partly raised and tail spread; male may also make vertical flight up to at least 6 m, then dive vertically to low branch; he also dances with body horizontal, tail fanned and slightly raised, nape feathers raised (showing dark blue collar), wings spread but tips drooped, and hops towards nest-site by pivoting along branch for up to 5 m before flying back and repeating the display; courtship of female involves provision by male of high-protein food items through egg-laying and incubation periods. Nest, built by female, mostly a cup of moss, dried grass, fine bark strips, plant fibres, leaves, animal hair and feathers, placed in hole or cleft in tree, or hole in post (including metal signpost) or other artificial site, e.g. wall , occasionally in old nest of other bird (or in foundations of large disused nest), rarely in hole in ground; nestboxes widely used; territory size usually within 1 ha, often much smaller, down to 0·16 ha. Clutch size varies geographically, altitudinally, with size of nest-cavity and quality of surrounding habitat, in Europe 7–13 eggs (clutches with in excess of 18 eggs generally the product of two females), in E of range generally 6–8 eggs; incubation by female, fed at nest by male, period 12–16 days; chicks fed by both parents, nesting period 16–23 days. Breeding success variable, influenced mostly by weather and predation, all following data from studies based on populations in nestboxes: in S England (Oxfordshire), 90–95% of young fledged in first three years, but success drastically reduced in following years through predation by weasel (Mustela nivalis); at second study site in S England, 33–77% of nests produced fledged young in different years and success affected mainly by grey squirrel (Sciurus carolinensis), weasels, rodents and Great Spotted Woodpecker (Dendrocopos major), and in some cases breeding pairs evicted by Tree Sparrows (Passer montanus); in Britain, success highest in gardens (86%), in Scots pine (Pinus sylvestris) plantations (71%) and in areas free from predation e.g. I of Rhum, in W Scotland (88%); nestling mortality rate affected by extent of rainfall, sudden drop in temperature, and availability of food, late-hatching young often poorly developed and suffer higher mortality. Juvenile mortality rate high in first three months of life, c. 70% between fledging and Nov. Breeds in first year. Estimated c. 75% of breeding pairs survive to following season (and some have remained together for 4 successive years); suburban pairs generally less productive, this attributed to lower nutritional value of available food. Maximum recorded longevity 12 years 4 months.
Conservation Status
Not globally threatened. Common to locally abundant, rare along N edge and in SE of range. Breeds occasionally outside normal limits of range, as e.g. in NE Syria (where otherwise a vagrant). European breeding population estimated at 16,500,000–23,000,000 pairs, most in Spain, British Is and Germany. Breeding densities vary according to habitat, provision of nestboxes and degree of competition from Parus major; densities generally highest in oak woodland, where can reach c. 250 pairs/km², and lowest in conifer plantations. Range gradually extended N in 20th century, to S Finland from c. 1900, particularly since mid-1950s (when also increased in Netherlands), and in 1970s expanded into Norway and N Scotland. Population in N Jordan first discovered in 1893, but not seen again until 1984, and breeding first proved in 1990. Appears able successfully to withstand severe winter weather, suffering only some local or short-term fluctuations in numbers; provision of food at birdtables considered to account for considerable portion of winter sustenance enabling large numbers to survive in times of hard or severe weather.
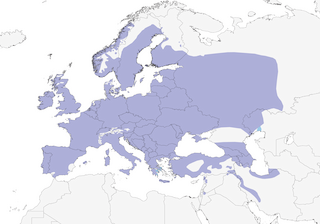
- Year-round
- Migration
- Breeding
- Non-Breeding































